Electron Mobility: Difference between revisions
MasterOfSwag (talk | contribs) No edit summary |
MasterOfSwag (talk | contribs) No edit summary |
||
| (4 intermediate revisions by the same user not shown) | |||
| Line 2: | Line 2: | ||
== | ==What is Electron Mobility?== | ||
Electron mobility is the characteristic of a metal or semiconductor, which predicts the velocity at which an electron will travel when influenced by an electric field. | Electron mobility is the characteristic of a metal or semiconductor, which predicts the velocity at which an electron will travel when influenced by an electric field. | ||
| Line 11: | Line 11: | ||
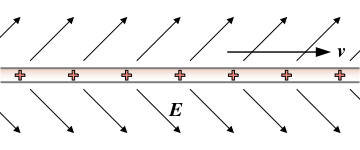
The drift velocity of electrons moving through a material, as a response to an electric field is designated as v, electron velocity. | The drift velocity of electrons moving through a material, as a response to an electric field is designated as v, electron velocity. | ||
== | [[File:efieldsssd.png]] | ||
==Factors== | |||
The electron mobility is defined in units of cm^2/(V·s). | The electron mobility is defined in units of cm^2/(V·s). | ||
| Line 26: | Line 28: | ||
[[File:aksk.png]] | [[File:aksk.png]] | ||
== | ==Applications and Fields== | ||
Higher mobility typically leads to better performance for semiconductors. | Higher mobility typically leads to better performance for semiconductors. | ||
| Line 38: | Line 37: | ||
Electron mobility of Silicon is 1400 cm^2/Vs | Electron mobility of Silicon is 1400 cm^2/Vs | ||
[[File: | [[File:siliconbla.jpg]] | ||
Comparatively, indium antimonide has a mobility of 77,000 cm^2/Vs | Comparatively, indium antimonide has a mobility of 77,000 cm^2/Vs | ||
| Line 46: | Line 45: | ||
At the University of Maryland, graphene has been recorded to have a mobility of 200,000 cm^2/Vs, and is also extremely thin. (How thin? Graphene is composed of a single layer of carbon atoms!) Such a combination could be extremely useful to the electronics industry, with electrons traveling 100 times faster in much less volume. | At the University of Maryland, graphene has been recorded to have a mobility of 200,000 cm^2/Vs, and is also extremely thin. (How thin? Graphene is composed of a single layer of carbon atoms!) Such a combination could be extremely useful to the electronics industry, with electrons traveling 100 times faster in much less volume. | ||
[[File: | [[File:graphenebla.jpg]] | ||
Electron mobility through a material is affected by external variables such as temperature. Increases in temperature will decrease the electron mobility by increasing frequency of collisions between electrons. | Electron mobility through a material is affected by external variables such as temperature. Increases in temperature will decrease the electron mobility by increasing frequency of collisions between electrons. | ||
Latest revision as of 00:43, 6 December 2015
claimed by djohnston35
What is Electron Mobility?
Electron mobility is the characteristic of a metal or semiconductor, which predicts the velocity at which an electron will travel when influenced by an electric field.
Electron mobility may also refer to a similar occurrence of charged particles in a liquid.
Note: Hole mobility is not the same concept as electron mobility and the numbers for these material properties will be different.
The drift velocity of electrons moving through a material, as a response to an electric field is designated as v, electron velocity.
Factors
The electron mobility is defined in units of cm^2/(V·s).
Mobility μ is a factor that impacts drift speed v through materials affected by an electric field E.
Conductivity σ in terms of electron mobility.
Current I in terms of charge density n, area of material A, charge q, and drift speed.
Applications and Fields
Higher mobility typically leads to better performance for semiconductors.
The electron mobility varies for different materials. A higher electron mobility will allow faster current flow and will allow electrical devices to be turned on and off more quickly. Electron mobility of Silicon is 1400 cm^2/Vs
Comparatively, indium antimonide has a mobility of 77,000 cm^2/Vs
At the University of Maryland, graphene has been recorded to have a mobility of 200,000 cm^2/Vs, and is also extremely thin. (How thin? Graphene is composed of a single layer of carbon atoms!) Such a combination could be extremely useful to the electronics industry, with electrons traveling 100 times faster in much less volume.
Electron mobility through a material is affected by external variables such as temperature. Increases in temperature will decrease the electron mobility by increasing frequency of collisions between electrons.
See also
References
http://news.softpedia.com/news/Electrons-100-times-Faster-in-Graphene-81534.shtml
http://phys.org/news/2008-03-physicists-electrons-faster-graphene-silicon.html
https://en.wikibooks.org/wiki/Materials_in_Electronics/Electrons_in_Conductors
https://en.wikibooks.org/wiki/Semiconductors/What_is_a_Semiconductor