Electromagnetic Radiation: Difference between revisions
No edit summary |
SaiGuvvala (talk | contribs) (Example of Calculating Energy of a Photon) |
||
| (82 intermediate revisions by 8 users not shown) | |||
| Line 1: | Line 1: | ||
Claimed by | '''Claimed by Venkata Guvvala (Sai)''' (Fall 2024) | ||
==Electromagnetic Radiation== | |||
===What is a Electromagnetic(EM) Radiation?=== | |||
Electromagnetic radiation is a form of energy that is all around us and takes many forms, such as radio waves, microwaves, infrared, visible light, ultraviolet, x-rays, and gamma rays. | |||
Before 1873, electricity and magnetism were thought to be two different forces. However, in 1873, Scottish Physicist James Maxwell developed his famous theory of electromagnetism. There are four main electro magnetic interactions according to Maxwell: | |||
* The force of attraction or repulsion between electric charges is inversely proportional to the square of the distance between them | |||
* Magnetic poles come in pairs that attract and repel each other much as electric charges do | |||
* An electric current in a wire produces a magnetic field whose direction depends on the direction of the current | |||
* A moving electric field produces a magnetic field, and vice versa | |||
== | ===General Properties=== | ||
Basic Description: Electromagnetic radiation can travel through empty space because it consists of oscillating electric and magnetic fields that sustain each other in the absence of a medium (meaning each field supports the existance of the other). Unlike mechanical waves, which rely on particles in a medium to propagate energy, electromagnetic waves can sustain iteself through Maxwell equations. This allows them to travel long distances in vacuum of space. | |||
The four Maxwell's Equations provide a complete description of possible spatial patterns of electric and magnetic field in space. | The four Maxwell's Equations provide a complete description of possible spatial patterns of electric and magnetic field in space. | ||
<div class="mw-collapsible-content"> | <div class="mw-collapsible-content"> | ||
| Line 12: | Line 23: | ||
*[[Faraday's Law]] | *[[Faraday's Law]] | ||
==The | Other than Maxwell's Four equations, there are general properties of all electromagnetic radiation: | ||
* Electromagnetic radiation can travel through empty space. Most other types of waves must travel through some sort of substance. For example, sound waves need either a gas, solid, or liquid to pass through in order to be heard | |||
* The speed of light is always a constant (3 x 10^8 m/s) | |||
* Wavelengths are measured between the distances of either crests or troughs. It is usually characterized by the Greek symbol λ (lambda). | |||
Electromagnetic waves are the self-propagating, mutual oscillation of electric and magnetic fields. The propagation of electromagnetic energy is often referred to as radiation. We can also say that the 'pulse' of these moving fields result in radiation (7). | |||
The equation for propagation is E=cB with c being the speed of light. This equation is derived from combining the two equations E=vB and B=u0e0vE, proving that v is equal to 3e8 meters/second. | |||
===Problem Solving Method and Equations=== | |||
To go about solving/analyzing mathematically an electromagnetic field using Maxwell's equations,this is how we proceed (7) | |||
*Establish the space and time in which the electric and magnetic fields are present | |||
*Check that Maxwell's equations can be applied in the situation above | |||
*Check when the charge accelerates, it produces these fields and therefore radiation | |||
*Show how these fields would interact with matter | |||
The equation of the Radiative Electric Field is: | |||
E= 1/(4πe0)*-qa/(c^2r) where a is the acceleration of the particle, c is the speed of light and r is the distance from the original location of the charge to right before the kink. This kink happens on the electric field because of the slight delay when the charge is moved. | |||
===Fields Made by Charges and Fields Made by Monopoles=== | |||
We can differentiate fields made by charges and the ones made by magnetic monopoles. (7) | |||
For fields made by charges, when the charge is | |||
*at rest, E=1/r^2 and B=0 | |||
*constant speed, E=1/r^2 and B=1/r^2 | |||
*accelerating, E=1/r and B=1/r | |||
For fields made by magnetic monopoles, the first point would have E and B switched. | |||
Theoretical Extra Info about Monopoles (field and charges made by monopoles): Magnetic monopoles are hypothetical particles made by grand unification theories and quantum mechanics models. Unlike electric charges, monopoles have not been observed. If they exist, their fields would mirror images of electric fields. Studying their interactions could provide insights into unification of fundamental forces. Particle accelerators and cosmic ray detection continue to search for such. | |||
==The EM Spectrum== | |||
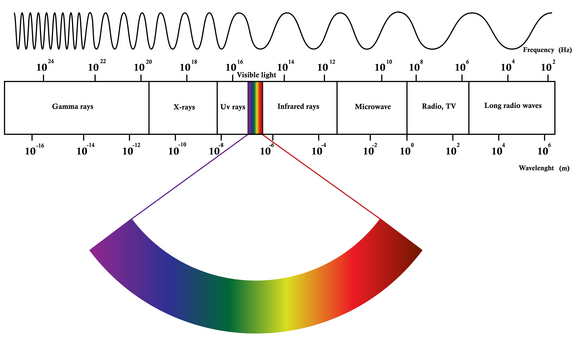
EM spectrum is a span of enormous range of wavelengths and frequencies. The EM spectrum is generally divided into 7 different regions, in order of decreasing wavelength and increasing energy and frequency. It ranges from Gamma rays to Long Radio Waves. Following are the lists of waves: | |||
* Gamma rays | |||
* X-rays | |||
* UV rays | |||
* Visible Light | |||
* Infrared Rays | |||
* Microwave | |||
* Radio, TV | |||
* Long radio waves | |||
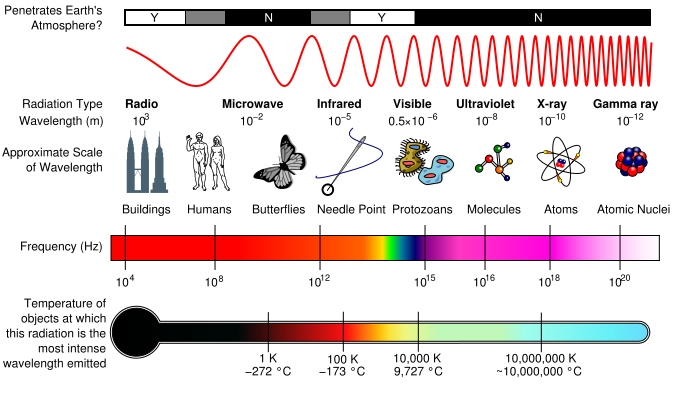
Different regions of EM spectrum interact with matter in unique ways because of their different energy levels. For instance, radio waves, having long wavelengths and low energy, pass through most materials without causing significant changes. Microwaves can excite water molecules, making them useful for heating. Ultraviolet rays, with higher energy, can break chemical bonds and are responsible for sunburn. X-rays and gamma rays, with even shorter wavelengths and higher energy, penetrate deeply into materials, making them useful for imaging bones and treating cancer. All of them have their uses and their own properties. We as humans can only observe small part. | |||
===A Mathematical Model | [[File:em-spectrum.jpg]] | ||
Although all these waves do different things, there is one thing in common : They all travel in waves. | |||
[[File:spectrum_Properties.png]] | |||
'''Infrared radiation''' can be released as heat or thermal energy. It can also be bounced back, which is called near infrared because of its similarities with visible light energy. Infrared Radiation is most commonly used in remote sensing as infrared sensors collect thermal energy, providing us with weather conditions. | |||
[[File:pic_snap_girl.png]] | |||
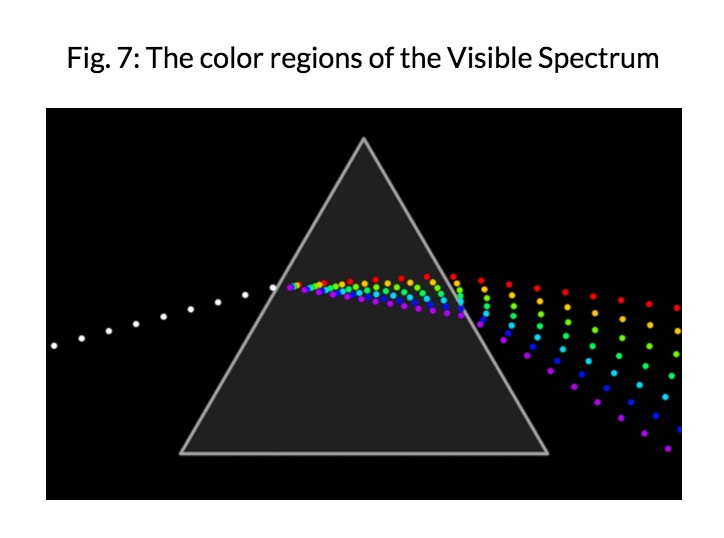
'''Visible Light''' is the only part of the electromagnetic spectrum that humans can see with a naked eye. This part of the spectrum includes a range of different colors that all represent a particular wavelength. Rainbows are formed in this way; light passes through matter in which it is absorbed or reflected based on its wavelength. As a result, some colors are reflected more than other, leading to the creation of a rainbow. | |||
[[File:pyramid123.jpg]] | |||
[[File:rainbow.jpg]] | |||
==Waves and Fields== | |||
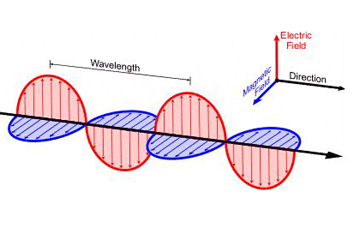
As we learned in class, electric field is produced when an electron is accelerating. Likewise, EM radiation is created when an atomic particle, like an electron, is accelerated by an electric field. The movement like this produces oscillating electric and magnetic fields, which travel at right angles to each other in a bundle of light energy called a photon. Photons travel in a harmonic wave at the fastest speed possible in the universe. | |||
[[File:waves image.jpg]] | |||
Electromagnetic waves are formed when an electric field couples with a magnetic field. Magnetic and electric fields of an electromagnetic wave are perpendicular to each other and to the direction of the wave. | |||
A wavelength (in m) is the distance between two consecutive peaks of a wave. Frequency is the number of waves that form in a given length of time. A wavelength and frequency are interrelated. A short wavelength indicates that the frequency will be higher because one cycle can pass in a shorter amount of time. Likewise, a longer wavelength has a lower frequency because each cycle takes longer to complete. | |||
[[File:waves_1.jpg]] | |||
Waves can be classified according to their nature: | |||
* Mechanical waves | |||
* Electromagnetic waves | |||
'''Mechanical Waves''' | |||
Mechanical waves require a medium (matter) to travel through. | |||
Examples are sound waves, water waves, ripples in strings or springs. | |||
''Water Waves'' | |||
[[File:waterwaves.gif]] | |||
''Sound Waves'' | |||
[[File:loudspeaker-waveform.gif]] | |||
'''Electromagnetic Waves''' | |||
Electromagnetic waves do not require a medium (matter) to travel through - they can travel through space. | |||
Examples are radio waves, visible light, x-rays. | |||
''X-RAYS'' | |||
[[File:x-rays.jpeg]] | |||
''Radio Waves'' | |||
[[File:facts-about-radio-waves.jpg]] | |||
''Visible Lights'' | |||
[[File:visible-spectrum123.jpg]] | |||
==A Mathematical Model== | |||
The position of the particle is defined by a sine wave: | The position of the particle is defined by a sine wave: | ||
'''y = ymaxsin(wt)''' | '''y = ymaxsin(wt)''' | ||
Where w is the angular frequency | |||
'''Amplitude''' | '''Amplitude''' | ||
The Amplitude of the sinusoidal Wave is the height of the peak in the wave measured from the zero line. | Amplitude is the distance from the maximum vertical displacement of the wave to the middle of the wave. The Amplitude of the sinusoidal Wave is the height of the peak in the wave measured from the zero line. This measures the magnitude of oscillation of a particular wave. The Amplitude is important because it tells you the intensity or brightness of a wave in comparison with other waves. | ||
'''Period''' | '''Period''' | ||
The period of the wave is the time between crests. | The period of the wave is the time between crests in seconds(s). | ||
T = 2pi/w-----(units of seconds) | T = 2pi/w-----(units of seconds) | ||
'''Frequency''' | '''Frequency''' | ||
Frequency is the number of cycles per second, and is expressed as sec-1 or Hertz(Hz). Frequency is directly proportional to energy and can be express as " | |||
'' E = hv '' | |||
where E is energy, h is Planck's constant ( 6.62607*10^-34J) and v is frequency | |||
f = 1/T | f = 1/T | ||
f = w/2pi----(Units Hertz) | f = w/2pi----(Units Hertz) | ||
'''Example of calculating energy: Calculating the Energy of a Photon''' | |||
Suppose we want to calculate the energy of a photon with a wavelength of 500 nm (visible light). | |||
Given: | |||
c = 3 * 10^8 m/s | |||
h = 6.626 * 10^-34 J*s | |||
λ = 500nm = 500 * 10^-9m | |||
v = c/λ = (3*10^8) / (5 * 10^-9) = 6 * 10^14 Hz | |||
Energy = hv = ((6.626×10^−34)(6×10^14) = 3.98 * 10^-19 J | |||
This energy corresponds to a green photon in the visible spectrum. | |||
'''Wavelength''' | '''Wavelength''' | ||
Wavelength is the distance between crests in meters. Wavelength is equal to the speed of light times frequency. Longer wavelength waves such as radio waves carry low energy; this is why we can listen to the radio without any harmful consequences. Shorter wavelength waves such as x-rays carry higher energy that can be hazardous to our health. | |||
[[File:shortlongwavelength.jpg]] | |||
'''Wavelength and Frequency''' | |||
The speed of light is the multiplication of the wavelength and frequency. | |||
''c=λν '' | |||
[[File:visible_EM_modes.png]] | |||
This diagram shows all properties of waves: | |||
[[File:wave_props.gif]] | |||
'''ENERGY FLUX''' | |||
Is defined by the following equation: | |||
S = (1/u0)*(E x B) in W/m^2 | |||
where B = E/c | |||
where c = speed of light | |||
[[File:energy_flux.gif]] | |||
==Connectedness: X-Rays== | |||
Electromagnetic Radiation while commonly thought of as only including visible light, radio waves, UV waves, and gamma rays; also include X-rays. In 1895, X-rays were initially discovered by William Roentgen, who accidentally fell upon the most important discovery about his life (Figure 1). Roentgen was already working on cathode rays, and because of a fluorescent glow that occurred during his experiments, covered his experimental apparatus with heavy black paper. However, when he did this, he discovered a glow coming from a screen several feet away. Through many more experiments, he discovered that a new type of energy, not cathode rays, were the cause of the glow. He named them “x-rays” and received the 1901 Nobel Prize in Physics. Roentgen never patented his monumental discovery and as a result, numerous researchers set out to find a multitude of uses and capitalize on his work. | |||
Primarily, people could now view objects that were hidden from plain view (i.e. scanners in airports). While X-rays are now used in 100’s of professions (security, chemistry, art galleries), its most important function is to view bones to determine abnormalities in humans. In fact, one of Roentgen’s first x-rays was of his wife’s hand (Figure 2). X-rays fall under the scope of electromagnetic radiation because, like all E.R. waves, it is comprised of photons. X-rays have wavelengths between 0.01 to 10 nanometers and fall between UV and Gamma Waves on the E.R. spectrum (Figure 3). | |||
There are two main methods in which an x-ray may be formed. Both require a vacuum-filled tube called an x-ray tube (Figure 4). With an anode on one end and a cathode on the other, an electric current is applied and a high energy electron is projected from the cathode, through the vacuum, and at the anode. In the characteristic x-ray generation approach, the electron from the cathode collides with an inner shell electron on an atom on the anode (Figure 5). Both of these electrons are ejected from the atom and an outer shell electron takes the place of the inner shell one. Because the outer electron must have a lower energy to fill the inner shell hole, it releases a photon with the equivalent energy of the difference between the two energy levels in the atom. This photon is the x-ray that is used to view objects such as bones. | |||
In the Bremsstrahlung x-ray generation method, the electron from the cathode is slowed as it passes the nucleus of an atom at the anode (Figure 6). As it slows and its path is changed, the loses energy (kinetic energy). This energy is also released as a photon which is subsequently called an x-ray. | |||
Depending on the voltage and current of the tube and the material of the anode, different types (as in wavelengths and energy) of x-rays can be produced and each one. However, all X-rays will continue to pass through objects until it reaches a material dense that stops it. However, density of the material required depends on the energy of the x-ray. For example, during a medical x-ray, x-rays of a certain energy will pass through soft tissue (skin, organs, etc) but not through bones. The x-rays that pass through the soft tissue will strike the screen and the absence of the x-rays absorbed by the bones will cause a negative space on the screen. The areas where x-rays do not strike will form the image of the bone. While the principles remain the same, x-ray machines today use incredible sophisticated technology to specify the type of x-ray they want and have greatly increased in accuracy since Roentgen’s initial discovery. | |||
Figure 1: | |||
[[File:Monali_Figure_1.jpg]] | |||
Figure 2: | |||
[[File:Monali_Figure_2.jpg]] | |||
Figure 3: | |||
[[File:Monali_Figure_3.png]] | |||
Figure 4: | |||
[[File:Monali_Figure_4.png]] | |||
Figure 5: | |||
[[File:Monali_Figure_5.png]] | |||
Figure 6: | |||
[[File:Monali_Figure_6.png]] | |||
*Information and photographs are pulled from references 1 through 5 cited below* | |||
==History== | |||
Already, during the Ancient Greek and Roman times, light was studied as the presence of deflection and refraction were noticed. | |||
Electromagnetic radiation of wavelengths in the early 19th century. The discovery of infrared radiation is ascribed to astronomer William Herschel, who published his results in 1800 before the Royal Society of London. Herschel used a glass Triangular prism (optics)|prism to refract light from the Sun and detected invisible rays that caused heating beyond the red part of the spectrum, through an increase in the temperature recorded with a thermometer. These "calorific rays" were later termed infrared. | |||
In 1801, Rohann Ritter, discovered the presence of ultraviolet light using salts. It was known that light could darken some silver halides and while doing so, he realized that the region beyond the violet bar (therefore ultraviolet) was more effective in changing the color of the halides. | |||
However,in 1864, while summarizing the theories of his time accumulating into his famous set of Maxwell equations, James Clerk Maxwell managed to deduce the speed of light being around 3e8 meters per second. This was instrumental in creating the rest of the spectrum. | |||
In 1887-1888 Physicist Heinrich Hertz not only tried to measure the velocity and frequency of electromagnetic radiation waves at other parts of the known spectrum of the time, but he was also able to prove that Maxwell's findings were correct. He did this on the microwave radiation as well. | |||
The discovery of X-rays occurred in 1895 by Wilhelm Rontgen when his barium platinocyanide detector screen began to glow under the presence of a discharge that passed through a cathode ray tube although the latter was completely covered. Once he determined its possible use, he tried to look at his wife's hand using this new discovery. However x-ray spectroscopy was not institutionalized until later by Karl Manne Siegbahn. | |||
In 1900, Paul Villard discovered Gamma rays although he initially thought that they were particles similar to alpha and beta particles which were emitted during radiation. These 'particles' were later proven to be part of the electromagnetic spectrum. | |||
===Practice Problems (new section by Zoila)=== | |||
Before we get to practicing some problems from this topic here are some helpful slides from class with equations and explanations.[[File:Thingy.jpg]] | |||
Here are some examples using these equations: | |||
[[File:Qo.jpg]] | |||
1.) | |||
==References== | |||
1. Elert, Glenn. "X-rays." X-rays – The Physics Hypertextbook. N.p., n.d. Web. 08 Apr. 2017. | |||
http://physics.info/x-ray/ | |||
2."X-rays." X-rays. N.p., n.d. Web. 08 Apr. 2017. http://www.physics.isu.edu/radinf/xray.htm | |||
3. "Basics of X-ray PhysicsX-ray production." Welcome to Radiology Masterclass. N.p., n.d. Web. 08 Apr. 2017. http://www.radiologymasterclass.co.uk/tutorials/physics/x-ray_physics_production#top_2nd_img | |||
4. "X-Rays." Image: Electromagnetic Spectrum. N.p., n.d. Web. 08 Apr. 2017. https://www.boundless.com/physics/textbooks/boundless-physics-textbook/electromagnetic-waves-23/the-electromagnetic-spectrum-165/x-rays-597-11175/images/electromagnetic-spectrum/ | |||
5. "This Month in Physics History." American Physical Society. N.p., n.d. Web. 08 Apr. 2017. https://www.aps.org/publications/apsnews/200111/history.cfm | |||
6. Editors, Spectroscopy. “The Electromagnetic Spectrum: A History.” Spectroscopy Home, 27 Oct. 2017, www.spectroscopyonline.com/electromagnetic-spectrum-history?id=&sk=&date=&&pageID=4. | |||
7. Chabay, Ruth W., and Bruce A. Sherwood. Matter & Interaction II: Electric & Magnetic Interactions, Version 1.2. John Wiley & Sons, 2003. | |||
Latest revision as of 23:52, 24 November 2024
Claimed by Venkata Guvvala (Sai) (Fall 2024)
Electromagnetic Radiation
What is a Electromagnetic(EM) Radiation?
Electromagnetic radiation is a form of energy that is all around us and takes many forms, such as radio waves, microwaves, infrared, visible light, ultraviolet, x-rays, and gamma rays.
Before 1873, electricity and magnetism were thought to be two different forces. However, in 1873, Scottish Physicist James Maxwell developed his famous theory of electromagnetism. There are four main electro magnetic interactions according to Maxwell:
- The force of attraction or repulsion between electric charges is inversely proportional to the square of the distance between them
- Magnetic poles come in pairs that attract and repel each other much as electric charges do
- An electric current in a wire produces a magnetic field whose direction depends on the direction of the current
- A moving electric field produces a magnetic field, and vice versa
General Properties
Basic Description: Electromagnetic radiation can travel through empty space because it consists of oscillating electric and magnetic fields that sustain each other in the absence of a medium (meaning each field supports the existance of the other). Unlike mechanical waves, which rely on particles in a medium to propagate energy, electromagnetic waves can sustain iteself through Maxwell equations. This allows them to travel long distances in vacuum of space.
The four Maxwell's Equations provide a complete description of possible spatial patterns of electric and magnetic field in space.
Other than Maxwell's Four equations, there are general properties of all electromagnetic radiation:
- Electromagnetic radiation can travel through empty space. Most other types of waves must travel through some sort of substance. For example, sound waves need either a gas, solid, or liquid to pass through in order to be heard
- The speed of light is always a constant (3 x 10^8 m/s)
- Wavelengths are measured between the distances of either crests or troughs. It is usually characterized by the Greek symbol λ (lambda).
Electromagnetic waves are the self-propagating, mutual oscillation of electric and magnetic fields. The propagation of electromagnetic energy is often referred to as radiation. We can also say that the 'pulse' of these moving fields result in radiation (7).
The equation for propagation is E=cB with c being the speed of light. This equation is derived from combining the two equations E=vB and B=u0e0vE, proving that v is equal to 3e8 meters/second.
Problem Solving Method and Equations
To go about solving/analyzing mathematically an electromagnetic field using Maxwell's equations,this is how we proceed (7)
- Establish the space and time in which the electric and magnetic fields are present
- Check that Maxwell's equations can be applied in the situation above
- Check when the charge accelerates, it produces these fields and therefore radiation
- Show how these fields would interact with matter
The equation of the Radiative Electric Field is: E= 1/(4πe0)*-qa/(c^2r) where a is the acceleration of the particle, c is the speed of light and r is the distance from the original location of the charge to right before the kink. This kink happens on the electric field because of the slight delay when the charge is moved.
Fields Made by Charges and Fields Made by Monopoles
We can differentiate fields made by charges and the ones made by magnetic monopoles. (7) For fields made by charges, when the charge is
- at rest, E=1/r^2 and B=0
- constant speed, E=1/r^2 and B=1/r^2
- accelerating, E=1/r and B=1/r
For fields made by magnetic monopoles, the first point would have E and B switched.
Theoretical Extra Info about Monopoles (field and charges made by monopoles): Magnetic monopoles are hypothetical particles made by grand unification theories and quantum mechanics models. Unlike electric charges, monopoles have not been observed. If they exist, their fields would mirror images of electric fields. Studying their interactions could provide insights into unification of fundamental forces. Particle accelerators and cosmic ray detection continue to search for such.
The EM Spectrum
EM spectrum is a span of enormous range of wavelengths and frequencies. The EM spectrum is generally divided into 7 different regions, in order of decreasing wavelength and increasing energy and frequency. It ranges from Gamma rays to Long Radio Waves. Following are the lists of waves:
- Gamma rays
- X-rays
- UV rays
- Visible Light
- Infrared Rays
- Microwave
- Radio, TV
- Long radio waves
Different regions of EM spectrum interact with matter in unique ways because of their different energy levels. For instance, radio waves, having long wavelengths and low energy, pass through most materials without causing significant changes. Microwaves can excite water molecules, making them useful for heating. Ultraviolet rays, with higher energy, can break chemical bonds and are responsible for sunburn. X-rays and gamma rays, with even shorter wavelengths and higher energy, penetrate deeply into materials, making them useful for imaging bones and treating cancer. All of them have their uses and their own properties. We as humans can only observe small part.
Although all these waves do different things, there is one thing in common : They all travel in waves.
Infrared radiation can be released as heat or thermal energy. It can also be bounced back, which is called near infrared because of its similarities with visible light energy. Infrared Radiation is most commonly used in remote sensing as infrared sensors collect thermal energy, providing us with weather conditions.
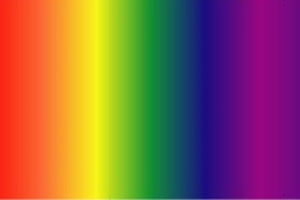
Visible Light is the only part of the electromagnetic spectrum that humans can see with a naked eye. This part of the spectrum includes a range of different colors that all represent a particular wavelength. Rainbows are formed in this way; light passes through matter in which it is absorbed or reflected based on its wavelength. As a result, some colors are reflected more than other, leading to the creation of a rainbow.
Waves and Fields
As we learned in class, electric field is produced when an electron is accelerating. Likewise, EM radiation is created when an atomic particle, like an electron, is accelerated by an electric field. The movement like this produces oscillating electric and magnetic fields, which travel at right angles to each other in a bundle of light energy called a photon. Photons travel in a harmonic wave at the fastest speed possible in the universe.
Electromagnetic waves are formed when an electric field couples with a magnetic field. Magnetic and electric fields of an electromagnetic wave are perpendicular to each other and to the direction of the wave.
A wavelength (in m) is the distance between two consecutive peaks of a wave. Frequency is the number of waves that form in a given length of time. A wavelength and frequency are interrelated. A short wavelength indicates that the frequency will be higher because one cycle can pass in a shorter amount of time. Likewise, a longer wavelength has a lower frequency because each cycle takes longer to complete.
Waves can be classified according to their nature:
- Mechanical waves
- Electromagnetic waves
Mechanical Waves
Mechanical waves require a medium (matter) to travel through. Examples are sound waves, water waves, ripples in strings or springs.
Water Waves

Sound Waves

Electromagnetic Waves
Electromagnetic waves do not require a medium (matter) to travel through - they can travel through space. Examples are radio waves, visible light, x-rays.
X-RAYS
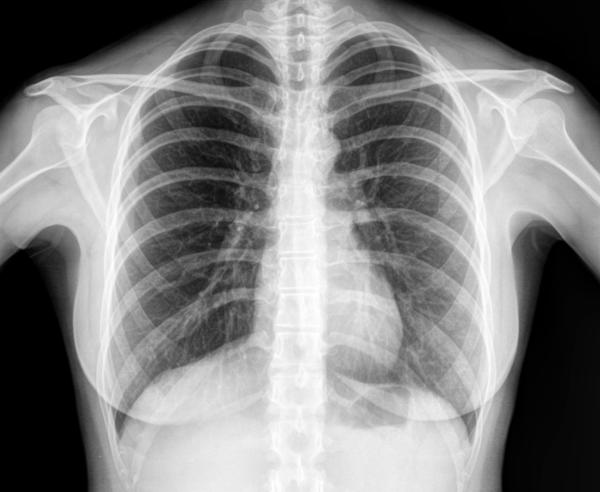
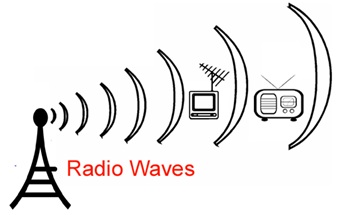
Radio Waves
Visible Lights
A Mathematical Model
The position of the particle is defined by a sine wave:
y = ymaxsin(wt)
Where w is the angular frequency
Amplitude
Amplitude is the distance from the maximum vertical displacement of the wave to the middle of the wave. The Amplitude of the sinusoidal Wave is the height of the peak in the wave measured from the zero line. This measures the magnitude of oscillation of a particular wave. The Amplitude is important because it tells you the intensity or brightness of a wave in comparison with other waves.
Period
The period of the wave is the time between crests in seconds(s).
T = 2pi/w-----(units of seconds)
Frequency
Frequency is the number of cycles per second, and is expressed as sec-1 or Hertz(Hz). Frequency is directly proportional to energy and can be express as "
E = hv where E is energy, h is Planck's constant ( 6.62607*10^-34J) and v is frequency
f = 1/T
f = w/2pi----(Units Hertz)
Example of calculating energy: Calculating the Energy of a Photon
Suppose we want to calculate the energy of a photon with a wavelength of 500 nm (visible light).
Given: c = 3 * 10^8 m/s h = 6.626 * 10^-34 J*s λ = 500nm = 500 * 10^-9m
v = c/λ = (3*10^8) / (5 * 10^-9) = 6 * 10^14 Hz
Energy = hv = ((6.626×10^−34)(6×10^14) = 3.98 * 10^-19 J This energy corresponds to a green photon in the visible spectrum.
Wavelength
Wavelength is the distance between crests in meters. Wavelength is equal to the speed of light times frequency. Longer wavelength waves such as radio waves carry low energy; this is why we can listen to the radio without any harmful consequences. Shorter wavelength waves such as x-rays carry higher energy that can be hazardous to our health.
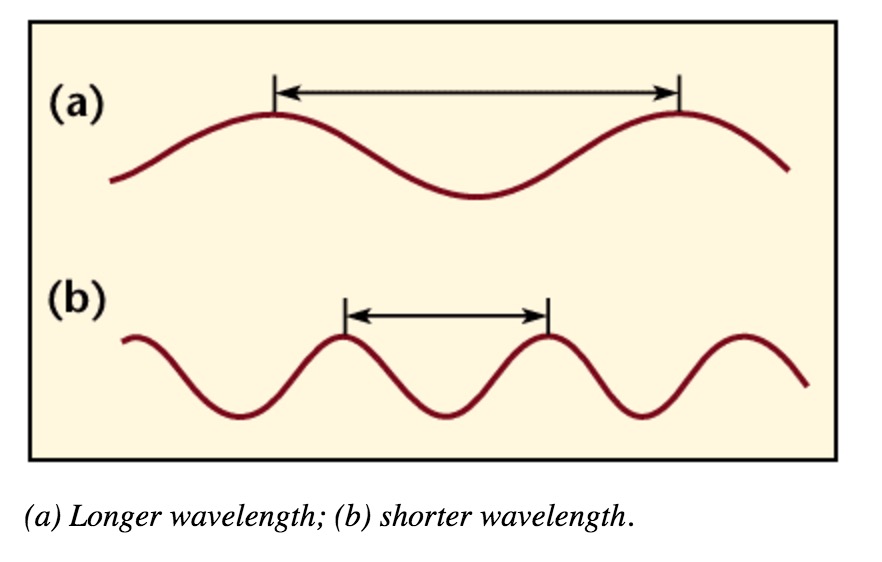
Wavelength and Frequency
The speed of light is the multiplication of the wavelength and frequency.
c=λν
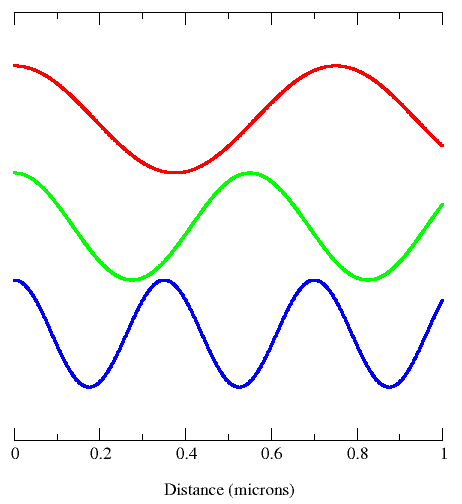
This diagram shows all properties of waves:

ENERGY FLUX
Is defined by the following equation:
S = (1/u0)*(E x B) in W/m^2
where B = E/c
where c = speed of light

Connectedness: X-Rays
Electromagnetic Radiation while commonly thought of as only including visible light, radio waves, UV waves, and gamma rays; also include X-rays. In 1895, X-rays were initially discovered by William Roentgen, who accidentally fell upon the most important discovery about his life (Figure 1). Roentgen was already working on cathode rays, and because of a fluorescent glow that occurred during his experiments, covered his experimental apparatus with heavy black paper. However, when he did this, he discovered a glow coming from a screen several feet away. Through many more experiments, he discovered that a new type of energy, not cathode rays, were the cause of the glow. He named them “x-rays” and received the 1901 Nobel Prize in Physics. Roentgen never patented his monumental discovery and as a result, numerous researchers set out to find a multitude of uses and capitalize on his work.
Primarily, people could now view objects that were hidden from plain view (i.e. scanners in airports). While X-rays are now used in 100’s of professions (security, chemistry, art galleries), its most important function is to view bones to determine abnormalities in humans. In fact, one of Roentgen’s first x-rays was of his wife’s hand (Figure 2). X-rays fall under the scope of electromagnetic radiation because, like all E.R. waves, it is comprised of photons. X-rays have wavelengths between 0.01 to 10 nanometers and fall between UV and Gamma Waves on the E.R. spectrum (Figure 3). There are two main methods in which an x-ray may be formed. Both require a vacuum-filled tube called an x-ray tube (Figure 4). With an anode on one end and a cathode on the other, an electric current is applied and a high energy electron is projected from the cathode, through the vacuum, and at the anode. In the characteristic x-ray generation approach, the electron from the cathode collides with an inner shell electron on an atom on the anode (Figure 5). Both of these electrons are ejected from the atom and an outer shell electron takes the place of the inner shell one. Because the outer electron must have a lower energy to fill the inner shell hole, it releases a photon with the equivalent energy of the difference between the two energy levels in the atom. This photon is the x-ray that is used to view objects such as bones.
In the Bremsstrahlung x-ray generation method, the electron from the cathode is slowed as it passes the nucleus of an atom at the anode (Figure 6). As it slows and its path is changed, the loses energy (kinetic energy). This energy is also released as a photon which is subsequently called an x-ray. Depending on the voltage and current of the tube and the material of the anode, different types (as in wavelengths and energy) of x-rays can be produced and each one. However, all X-rays will continue to pass through objects until it reaches a material dense that stops it. However, density of the material required depends on the energy of the x-ray. For example, during a medical x-ray, x-rays of a certain energy will pass through soft tissue (skin, organs, etc) but not through bones. The x-rays that pass through the soft tissue will strike the screen and the absence of the x-rays absorbed by the bones will cause a negative space on the screen. The areas where x-rays do not strike will form the image of the bone. While the principles remain the same, x-ray machines today use incredible sophisticated technology to specify the type of x-ray they want and have greatly increased in accuracy since Roentgen’s initial discovery.
- Information and photographs are pulled from references 1 through 5 cited below*
History
Already, during the Ancient Greek and Roman times, light was studied as the presence of deflection and refraction were noticed. Electromagnetic radiation of wavelengths in the early 19th century. The discovery of infrared radiation is ascribed to astronomer William Herschel, who published his results in 1800 before the Royal Society of London. Herschel used a glass Triangular prism (optics)|prism to refract light from the Sun and detected invisible rays that caused heating beyond the red part of the spectrum, through an increase in the temperature recorded with a thermometer. These "calorific rays" were later termed infrared.
In 1801, Rohann Ritter, discovered the presence of ultraviolet light using salts. It was known that light could darken some silver halides and while doing so, he realized that the region beyond the violet bar (therefore ultraviolet) was more effective in changing the color of the halides. However,in 1864, while summarizing the theories of his time accumulating into his famous set of Maxwell equations, James Clerk Maxwell managed to deduce the speed of light being around 3e8 meters per second. This was instrumental in creating the rest of the spectrum.
In 1887-1888 Physicist Heinrich Hertz not only tried to measure the velocity and frequency of electromagnetic radiation waves at other parts of the known spectrum of the time, but he was also able to prove that Maxwell's findings were correct. He did this on the microwave radiation as well.
The discovery of X-rays occurred in 1895 by Wilhelm Rontgen when his barium platinocyanide detector screen began to glow under the presence of a discharge that passed through a cathode ray tube although the latter was completely covered. Once he determined its possible use, he tried to look at his wife's hand using this new discovery. However x-ray spectroscopy was not institutionalized until later by Karl Manne Siegbahn.
In 1900, Paul Villard discovered Gamma rays although he initially thought that they were particles similar to alpha and beta particles which were emitted during radiation. These 'particles' were later proven to be part of the electromagnetic spectrum.
Practice Problems (new section by Zoila)
Before we get to practicing some problems from this topic here are some helpful slides from class with equations and explanations.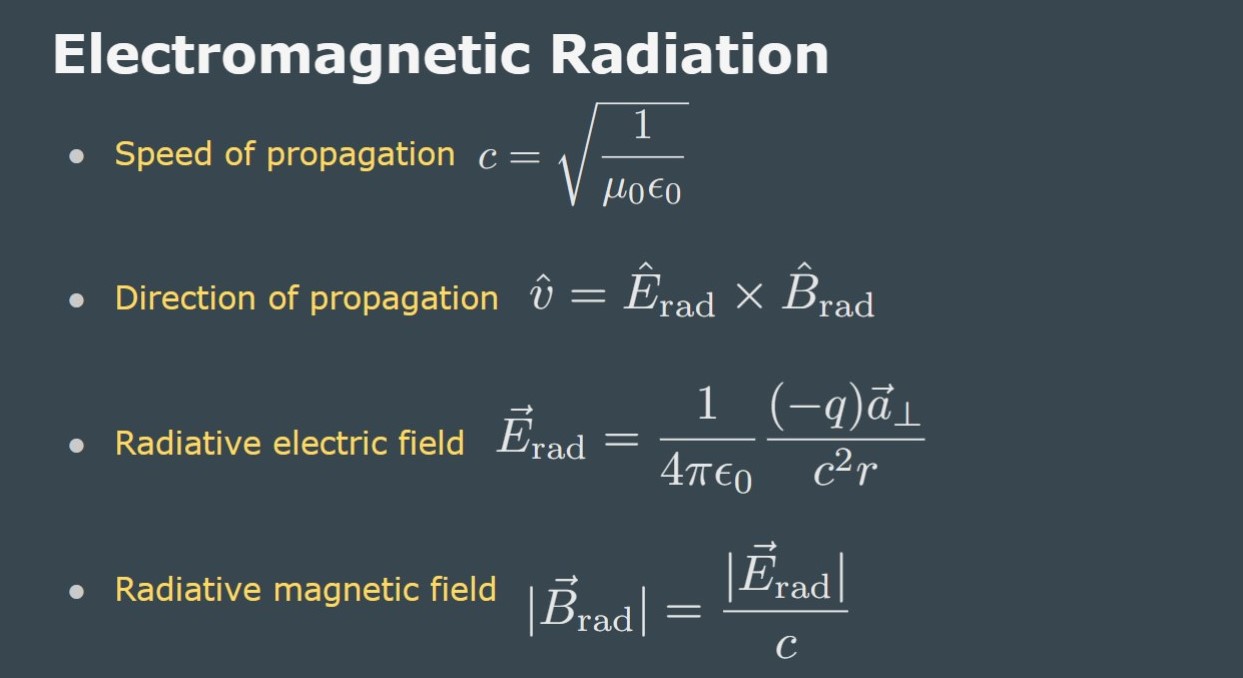
Here are some examples using these equations:

1.)
References
1. Elert, Glenn. "X-rays." X-rays – The Physics Hypertextbook. N.p., n.d. Web. 08 Apr. 2017. http://physics.info/x-ray/
2."X-rays." X-rays. N.p., n.d. Web. 08 Apr. 2017. http://www.physics.isu.edu/radinf/xray.htm
3. "Basics of X-ray PhysicsX-ray production." Welcome to Radiology Masterclass. N.p., n.d. Web. 08 Apr. 2017. http://www.radiologymasterclass.co.uk/tutorials/physics/x-ray_physics_production#top_2nd_img
4. "X-Rays." Image: Electromagnetic Spectrum. N.p., n.d. Web. 08 Apr. 2017. https://www.boundless.com/physics/textbooks/boundless-physics-textbook/electromagnetic-waves-23/the-electromagnetic-spectrum-165/x-rays-597-11175/images/electromagnetic-spectrum/
5. "This Month in Physics History." American Physical Society. N.p., n.d. Web. 08 Apr. 2017. https://www.aps.org/publications/apsnews/200111/history.cfm
6. Editors, Spectroscopy. “The Electromagnetic Spectrum: A History.” Spectroscopy Home, 27 Oct. 2017, www.spectroscopyonline.com/electromagnetic-spectrum-history?id=&sk=&date=&&pageID=4.
7. Chabay, Ruth W., and Bruce A. Sherwood. Matter & Interaction II: Electric & Magnetic Interactions, Version 1.2. John Wiley & Sons, 2003.