Conservation of Energy: Difference between revisions
Kylebarber (talk | contribs) No edit summary |
Tag: Manual revert |
||
| (104 intermediate revisions by 6 users not shown) | |||
| Line 1: | Line 1: | ||
<div class="center" style="width: auto; margin-left: auto; margin-right: auto;">'''Editor: Ethan Nguyen-Tu, Spring 2023'''</div> | |||
'''What is the Law of Conservation of Energy?''' | |||
<br> | <br> | ||
The Law of Conservation of Energy states that energy cannot be created or destroyed, but it can change from one form of energy to another. | |||
<br> | <br> | ||
==Main Ideas== | ==Main Ideas== | ||
* | * The law of conservation of energy states that the '''total amount of energy''' of a system '''before and after''' an interaction between objects in '''conserved'''. | ||
* This only applies to isolated systems (no outside forces acting on the system). | * This only applies to '''isolated systems''' (no outside forces acting on the system). | ||
** Not Isolated: An object sliding across a rough floor (system = the object). There is work being done by the floor on the object because of the frictional force. | ** Not Isolated: An object sliding across a rough floor (system = the object). There is work being done by the floor on the object because of the frictional force. Energy lost to heat due to friction is an example of mechanical energy being converted into thermal energy. | ||
** Isolated: An object sliding across a rough floor (system = the object AND the floor). There is no work done on the system because all the forces are contained in the system. | ** Isolated: An object sliding across a rough floor (system = the object AND the floor). There is no work done on the system because all the forces are contained in the system. | ||
[[File:canonfire.jpg|800px|center]] | |||
===What does it mean?=== | |||
Since The Law of Conservation of Energy states energy cannot be created or destroyed, this means that the total energy in the universe is constant and does not change in value, assuming there is nothing beyond the universe. | |||
<br> | |||
:Therefore, in equation format: | |||
::[e.1] <math>E_{total, universe} = \text{Some Constant}</math> | |||
::[e.2] <math>\Delta E_{total, universe} = 0</math> | |||
==Mathematical Model== | |||
===Conservation of Energy=== | |||
::[e.1] <math> E_{total, universe} = \text{Some Constant} </math> | |||
::[e.2] <math> \Delta E_{total, universe} = 0 </math> | |||
===Conservation of Energy & [[The Energy Principle]]=== | |||
[[ | ::[e.3] <math> \Delta E_{system} + \Delta E_{surroundings} = 0 </math> | ||
===Isolated System=== | |||
< | * <b> ΔE = W + Q </b> (if no heat transfer indicated, Q = 0; if no external forces acting on system, W = 0) | ||
* <b> ΔE = K + U </b> (The total energy is the sum of the kinetic and potential energies. From this, you can infer that for an isolated system, any change in kinetic energy will correspond in an equal but opposite change in the potential energy and vice versa.) | |||
These formulas can be interchanged. For example, if you know work and heat transfer are zero, energy equals zero, so K + U will equal zero | |||
== | [https://www.youtube.com/watch?v=kw_4Loo1HR4 Basic Explanation of Conservation of Energy] | ||
<br> | |||
[https://www.youtube.com/watch?v=EZrJNIBX2wk Skater Visualization of Transfers of Energy] | |||
===Computational Model=== | |||
These gifs demonstrate the energy principal from a '''Conservation of Energy''' standpoint. As the ball on a spring approaches the equilibrium point, the '''kinetic energy increases''' and the '''spring potential decreases'''. These values will '''oscillate''', but the '''total energy will stay constant'''! This demonstration was written in GlowScript and '''iteratively updates the ball's momentum''' while taking into account the spring force. | |||
[[File:Spring1.gif|300px]] | |||
[[File:Graphspring.gif]] | |||
===Useful Energy Formulas=== | |||
[[ | '''[[Kinetic Energy]]''' | ||
< | * Kinetic Energy: <math>{E_{kinetic} = K = \frac{1}{2}m\overrightarrow{v}^2}</math> | ||
=== | '''[[Potential Energy]]''' | ||
* Gravitational Potential: <math>{U_{gravity} = U_g = \frac{Gm_1m_2}{ \big{|} \overrightarrow{r} \big{|}}(-\hat{r})}</math> | |||
** Approximation when near the Earth's Surface: <math>{\Delta U_g=mg\Delta h}</math> | |||
* Spring (Elastic) Potential: <math>{U_{spring} = U_s = \frac{1}{2}k_ss^2}</math> | |||
[ | '''[[Thermal Energy]]''' | ||
* Thermal Energy: <math>{\Delta E_{thermal} = mC \Delta T }</math> | |||
==Examples== | ==Examples== | ||
To view the solution, click '''Expand''' at the top right of each question. | |||
===Easy=== | |||
<div class="toccolours mw-collapsible mw-collapsed"> | |||
====Question 1==== | |||
A ball is at rest on a table with 50 J of potential energy. It then rolls off the table, and at one point in time as it falls, the ball has 30 J of kinetic energy. | |||
'''What is the potential energy of the ball at that instant?''' | |||
= | <div class="mw-collapsible-content"> | ||
[[File:EasyEnergyConservation.png]] | [[File:EasyEnergyConservation.png]] | ||
<br>See Reference 3 | <br> | ||
See Reference 3 | |||
E<sub>initial</sub> = E<sub>final</sub> <br> | |||
K<sub>initial</sub> + U<sub>initial</sub> = K<sub>final</sub> + U<sub>final</sub> <br> | K<sub>initial</sub> + U<sub>initial</sub> = K<sub>final</sub> + U<sub>final</sub> <br> | ||
0 J + 50 J = 30 J + U<sub>final</sub> <br> | 0 J + 50 J = 30 J + U<sub>final</sub> <br> | ||
U<sub>final</sub> = 20 J </ | U<sub>final</sub> = 20 J | ||
</div> | |||
</div> | |||
===Medium=== | |||
<div class="toccolours mw-collapsible mw-collapsed"> | |||
====Question 1==== | |||
A ball is at rest 50 m above the ground. You then drop the ball. | |||
'''What is its speed before hitting the ground?''' | |||
<div class="mw-collapsible-content"> | |||
[[File:ballcalc.jpg|400px]] | |||
</div> | |||
</div> | |||
< | |||
===Hard=== | |||
<div class="toccolours mw-collapsible mw-collapsed"> | |||
====Question 1==== | |||
The driver of an SUV (m = 1700 kg) isn’t paying attention and rear ends a car (m = 950 kg) on level ground at a red light. On impact, both drivers lock their brakes. The SUV and car stick together and travel a distance of 8.2 m before they come to a stop. The coefficient of friction between the tires and the road is 0.72. | |||
'''How fast was the SUV traveling just before the collision?''' | |||
< | <div class="mw-collapsible-content"> | ||
[[File:collidecars.jpg|600px]] | |||
</div> | |||
</div> | |||
==Connectedness== | ==Connectedness== | ||
===How does it relate to things we want to study?=== | |||
This | In Physics, we separate what we are looking at into a system and its surroundings. This is a zero-sum separation where what we are interested in is included in the system and everything else in the universe is lumped into the system's surroundings. | ||
<br> | |||
: In equation format: Universe = System + Surroundings [e.4] | |||
This division of the universe into a system and its surroundings can also be applied to the total energy in the universe. | |||
<br> | |||
: In equation format: <math>E_{total, universe} = E_{system} + E_{surroundings} </math> [e.5] | |||
Furthermore, the Law of Conservation of Energy tells us that the total energy in the universe is constant. | |||
: In equation format: <math>E_{total, universe} = E_{system} + E_{surroundings} = \text{Some Constant} </math> [e.6] | |||
However, when considering the surroundings in [e.5] and [e.6] for systems that are not the entire universe, we run into an important problem: | |||
<br> | |||
: When considering the system's surroundings, is that it is highly time inefficient, and essentially impossible, to consider and factor in everything in the universe that is not included in the system. Therefore, this means that we can only find completely accurate energy values by applying the Law of Conservation of Energy to isolated systems in which the system has no net external force and does not exchange matter and energy with its surroundings. In this case, the Law of Conservation of Energy tells us that the energy within the isolated system is constant. Nevertheless, applying the Law of Conservation of Energy to closed systems, in which matter is not able to enter or leave but energy can, we can get close approximations by identifying the main actors in the surroundings that influence our system. | |||
===How does it related to [[The Energy Principle]]?=== | |||
Since the Law of Conservation of Energy says energy cannot be created or destroyed, [[The Energy Principle]] tells us that the only way for a system to gain or lose energy is from its surroundings losing or gaining the same amount of energy. Therefore, [[The Energy Principle]] can be generalized in terms of conservation of energy. | |||
<br> | |||
: Written in equation form, [[The Energy Principle]] in terms of the Law of Conservation of Energy is: <math>{\Delta E_{system}+\Delta E_{surroundings}=0}</math> [e.3] | |||
==History== | ==History== | ||
<b>Who:</b> Many physicists contributed to the knowledge of energy, however it is most notably attributed to Julius Robert Mayer | |||
<br> | |||
<br><b>What:</b> Most formally discovered the law of conservation of energy | <b>What:</b> Most formally discovered the law of conservation of energy | ||
<br><b>When:</b> 1842 | <br> | ||
<br><b>Where:</b> Germany | <b>When:</b> 1842 | ||
<br><b>Why:</b> To explain what happens to energy in an isolated system | <br> | ||
<br>See Reference 6 | <b>Where:</b> Germany | ||
<br> | |||
<b>Why:</b> To explain what happens to energy in an isolated system | |||
<br> | |||
See Reference 6 | |||
== See also == | == See also == | ||
[[Kinetic Energy]] | * [[The Energy Principle]] | ||
[[Potential Energy]] | * [[Kinetic Energy]] | ||
[[Work]] | * [[Potential Energy]] | ||
* [[Work]] | |||
Goldstein, Martin, and Inge F., (1993). The Refrigerator and the Universe. Harvard Univ. Press. A gentle introduction. | ===Additional Reading=== | ||
Kroemer, Herbert; Kittel, Charles (1980). Thermal Physics (2nd ed.). W. H. Freeman Company. ISBN 0-7167-1088-9. | # Goldstein, Martin, and Inge F., (1993). The Refrigerator and the Universe. Harvard Univ. Press. A gentle introduction. | ||
Nolan, Peter J. (1996). Fundamentals of College Physics, 2nd ed. William C. Brown Publishers. | # Kroemer, Herbert; Kittel, Charles (1980). Thermal Physics (2nd ed.). W. H. Freeman Company. ISBN 0-7167-1088-9. | ||
# Nolan, Peter J. (1996). Fundamentals of College Physics, 2nd ed. William C. Brown Publishers. | |||
===External | ===External Links=== | ||
[http://www.physnet.org/modules/pdf_modules/m158.pdf The First Law of Thermodynamics] | # [https://www.khanacademy.org/science/physics/work-and-energy/work-and-energy-tutorial/a/what-is-conservation-of-energy Khan Academy] | ||
# [http://www.physicsclassroom.com/class/energy/Lesson-2/Application-and-Practice-Questions Practice Questions] | |||
# [http://physics.info/energy-conservation/problems.shtml More Practice] | |||
# [http://gilliesphysics.weebly.com/uploads/5/7/5/2/57520801/conservation_of_energy_practice_problems.pdf Basic Examples] | |||
# [http://www.physnet.org/modules/pdf_modules/m158.pdf The First Law of Thermodynamics] | |||
==References== | ==References== | ||
# "Conservation of Energy." Hmolpedia. Web. 1 Dec. 2015. <http://www.eoht.info/page/Conservation+of+energy>. | |||
# "University of Wisconsin Green Bay." Speed & Stopping Distance of a Roller-Coaster. Web. 1 Dec. 2015. <http://www.uwgb.edu/fenclh/problems/energy/2/>. | |||
# "Motion." G9 to Engineering. Web. 1 Dec. 2015. <http://www.g9toengineering.com/resources/translational.htm>. | |||
# "Energy of Falling Object." HyperPhysics. Web. 1 Dec. 2015. <http://hyperphysics.phy-astr.gsu.edu/hbase/flobj.html>. | |||
# "Conservation of Energy & Momentum Problem: Collision of Two Cars at a Stoplight." University of Wisconsin- Green Bay Physics. Web. 2 Dec. 2015. <http://www.uwgb.edu/fenclh/problems/energy/6/>. | |||
# "Law of Conservation of Mass Energy." Law of Conservation of Mass Energy. Web. 3 Dec. 2015. <http://www.chemteam.info/Thermochem/Law-Cons-Mass-Energy.html>. | |||
# "Law of Conservation of Energy" New York University. Web. 18 Apr. 2018. <http://www.nyu.edu/classes/tuckerman/adv.chem/lectures/lecture_2/node4.html>. | |||
# "Law of Conversation of Energy" ME Mechanical. Web. 18 Apr. 2018. <https://me-mechanicalengineering.com/law-of-conservation-of-energy/>. | |||
# "Law of conservation of energy" University of Calgary. Web. 8 Apr. 2023. <https://energyeducation.ca/encyclopedia/Law_of_conservation_of_energy>. | |||
# "Basic Definitions" LibreTexts Chemistry. Web. 8 Apr. 2023. <https://chem.libretexts.org/Bookshelves/General_Chemistry/General_Chemistry_Supplement_(Eames)/Thermochemistry/Basic_Definitions#:~:text=A%20closed%20system%20does%20not,is%20approximately%20an%20isolated%20system.> | |||
[[Category:Energy]] | [[Category:Energy]] | ||
Latest revision as of 20:10, 16 April 2023
What is the Law of Conservation of Energy?
The Law of Conservation of Energy states that energy cannot be created or destroyed, but it can change from one form of energy to another.
Main Ideas
- The law of conservation of energy states that the total amount of energy of a system before and after an interaction between objects in conserved.
- This only applies to isolated systems (no outside forces acting on the system).
- Not Isolated: An object sliding across a rough floor (system = the object). There is work being done by the floor on the object because of the frictional force. Energy lost to heat due to friction is an example of mechanical energy being converted into thermal energy.
- Isolated: An object sliding across a rough floor (system = the object AND the floor). There is no work done on the system because all the forces are contained in the system.
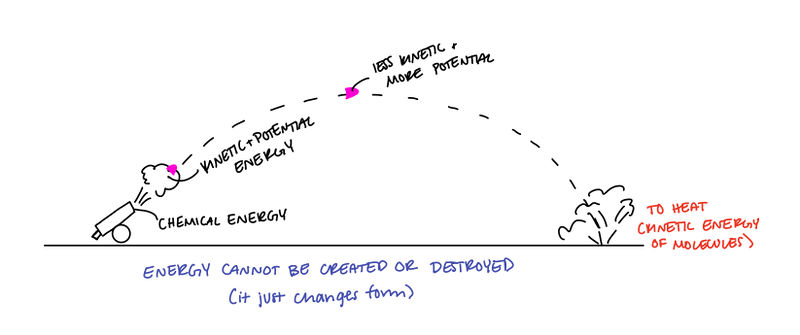
What does it mean?
Since The Law of Conservation of Energy states energy cannot be created or destroyed, this means that the total energy in the universe is constant and does not change in value, assuming there is nothing beyond the universe.
- Therefore, in equation format:
- [e.1] [math]\displaystyle{ E_{total, universe} = \text{Some Constant} }[/math]
- [e.2] [math]\displaystyle{ \Delta E_{total, universe} = 0 }[/math]
Mathematical Model
Conservation of Energy
- [e.1] [math]\displaystyle{ E_{total, universe} = \text{Some Constant} }[/math]
- [e.2] [math]\displaystyle{ \Delta E_{total, universe} = 0 }[/math]
Conservation of Energy & The Energy Principle
- [e.3] [math]\displaystyle{ \Delta E_{system} + \Delta E_{surroundings} = 0 }[/math]
Isolated System
- ΔE = W + Q (if no heat transfer indicated, Q = 0; if no external forces acting on system, W = 0)
- ΔE = K + U (The total energy is the sum of the kinetic and potential energies. From this, you can infer that for an isolated system, any change in kinetic energy will correspond in an equal but opposite change in the potential energy and vice versa.)
These formulas can be interchanged. For example, if you know work and heat transfer are zero, energy equals zero, so K + U will equal zero
Basic Explanation of Conservation of Energy
Skater Visualization of Transfers of Energy
Computational Model
These gifs demonstrate the energy principal from a Conservation of Energy standpoint. As the ball on a spring approaches the equilibrium point, the kinetic energy increases and the spring potential decreases. These values will oscillate, but the total energy will stay constant! This demonstration was written in GlowScript and iteratively updates the ball's momentum while taking into account the spring force.
Useful Energy Formulas
- Kinetic Energy: [math]\displaystyle{ {E_{kinetic} = K = \frac{1}{2}m\overrightarrow{v}^2} }[/math]
- Gravitational Potential: [math]\displaystyle{ {U_{gravity} = U_g = \frac{Gm_1m_2}{ \big{|} \overrightarrow{r} \big{|}}(-\hat{r})} }[/math]
- Approximation when near the Earth's Surface: [math]\displaystyle{ {\Delta U_g=mg\Delta h} }[/math]
- Spring (Elastic) Potential: [math]\displaystyle{ {U_{spring} = U_s = \frac{1}{2}k_ss^2} }[/math]
- Thermal Energy: [math]\displaystyle{ {\Delta E_{thermal} = mC \Delta T } }[/math]
Examples
To view the solution, click Expand at the top right of each question.
Easy
Question 1
A ball is at rest on a table with 50 J of potential energy. It then rolls off the table, and at one point in time as it falls, the ball has 30 J of kinetic energy.
What is the potential energy of the ball at that instant?
Medium
Question 1
A ball is at rest 50 m above the ground. You then drop the ball.
What is its speed before hitting the ground?
Hard
Question 1
The driver of an SUV (m = 1700 kg) isn’t paying attention and rear ends a car (m = 950 kg) on level ground at a red light. On impact, both drivers lock their brakes. The SUV and car stick together and travel a distance of 8.2 m before they come to a stop. The coefficient of friction between the tires and the road is 0.72.
How fast was the SUV traveling just before the collision?
Connectedness
How does it relate to things we want to study?
In Physics, we separate what we are looking at into a system and its surroundings. This is a zero-sum separation where what we are interested in is included in the system and everything else in the universe is lumped into the system's surroundings.
- In equation format: Universe = System + Surroundings [e.4]
This division of the universe into a system and its surroundings can also be applied to the total energy in the universe.
- In equation format: [math]\displaystyle{ E_{total, universe} = E_{system} + E_{surroundings} }[/math] [e.5]
Furthermore, the Law of Conservation of Energy tells us that the total energy in the universe is constant.
- In equation format: [math]\displaystyle{ E_{total, universe} = E_{system} + E_{surroundings} = \text{Some Constant} }[/math] [e.6]
However, when considering the surroundings in [e.5] and [e.6] for systems that are not the entire universe, we run into an important problem:
- When considering the system's surroundings, is that it is highly time inefficient, and essentially impossible, to consider and factor in everything in the universe that is not included in the system. Therefore, this means that we can only find completely accurate energy values by applying the Law of Conservation of Energy to isolated systems in which the system has no net external force and does not exchange matter and energy with its surroundings. In this case, the Law of Conservation of Energy tells us that the energy within the isolated system is constant. Nevertheless, applying the Law of Conservation of Energy to closed systems, in which matter is not able to enter or leave but energy can, we can get close approximations by identifying the main actors in the surroundings that influence our system.
Since the Law of Conservation of Energy says energy cannot be created or destroyed, The Energy Principle tells us that the only way for a system to gain or lose energy is from its surroundings losing or gaining the same amount of energy. Therefore, The Energy Principle can be generalized in terms of conservation of energy.
- Written in equation form, The Energy Principle in terms of the Law of Conservation of Energy is: [math]\displaystyle{ {\Delta E_{system}+\Delta E_{surroundings}=0} }[/math] [e.3]
History
Who: Many physicists contributed to the knowledge of energy, however it is most notably attributed to Julius Robert Mayer
What: Most formally discovered the law of conservation of energy
When: 1842
Where: Germany
Why: To explain what happens to energy in an isolated system
See Reference 6
See also
Additional Reading
- Goldstein, Martin, and Inge F., (1993). The Refrigerator and the Universe. Harvard Univ. Press. A gentle introduction.
- Kroemer, Herbert; Kittel, Charles (1980). Thermal Physics (2nd ed.). W. H. Freeman Company. ISBN 0-7167-1088-9.
- Nolan, Peter J. (1996). Fundamentals of College Physics, 2nd ed. William C. Brown Publishers.
External Links
References
- "Conservation of Energy." Hmolpedia. Web. 1 Dec. 2015. <http://www.eoht.info/page/Conservation+of+energy>.
- "University of Wisconsin Green Bay." Speed & Stopping Distance of a Roller-Coaster. Web. 1 Dec. 2015. <http://www.uwgb.edu/fenclh/problems/energy/2/>.
- "Motion." G9 to Engineering. Web. 1 Dec. 2015. <http://www.g9toengineering.com/resources/translational.htm>.
- "Energy of Falling Object." HyperPhysics. Web. 1 Dec. 2015. <http://hyperphysics.phy-astr.gsu.edu/hbase/flobj.html>.
- "Conservation of Energy & Momentum Problem: Collision of Two Cars at a Stoplight." University of Wisconsin- Green Bay Physics. Web. 2 Dec. 2015. <http://www.uwgb.edu/fenclh/problems/energy/6/>.
- "Law of Conservation of Mass Energy." Law of Conservation of Mass Energy. Web. 3 Dec. 2015. <http://www.chemteam.info/Thermochem/Law-Cons-Mass-Energy.html>.
- "Law of Conservation of Energy" New York University. Web. 18 Apr. 2018. <http://www.nyu.edu/classes/tuckerman/adv.chem/lectures/lecture_2/node4.html>.
- "Law of Conversation of Energy" ME Mechanical. Web. 18 Apr. 2018. <https://me-mechanicalengineering.com/law-of-conservation-of-energy/>.
- "Law of conservation of energy" University of Calgary. Web. 8 Apr. 2023. <https://energyeducation.ca/encyclopedia/Law_of_conservation_of_energy>.
- "Basic Definitions" LibreTexts Chemistry. Web. 8 Apr. 2023. <https://chem.libretexts.org/Bookshelves/General_Chemistry/General_Chemistry_Supplement_(Eames)/Thermochemistry/Basic_Definitions#:~:text=A%20closed%20system%20does%20not,is%20approximately%20an%20isolated%20system.>




