Electronic Energy Levels and Photons: Difference between revisions
No edit summary |
Zach Shapiro (talk | contribs) No edit summary |
||
| (53 intermediate revisions by 3 users not shown) | |||
| Line 1: | Line 1: | ||
Zachary Shapiro Spring 2022 | |||
= | In a classical Bohr model, electrons have discrete quantities of energy, called energy levels, as they orbit the central nucleus. An atom is thought to contain several "shells" in which electrons orbit the nucleus, and each shell represents a different energy level. Which shell an electron is currently in is denoted by the principal quantum number (n = 1,2,3...). Lower principal quantum numbers represent an electron shell that has a lower energy level and is closer to the nucleus. | ||
Electrons can transfer to a higher energy level by absorbing energy (usually in the form of a photon). However, the electron will eventually return to its ground state, releasing a photon in the process. Photons are the fundamental unit of electromagnetic radiation and are responsible for the phenomenon of light. They are considered to be massless and thus travel at the speed of light (299,792,458 m/s). | |||
Electrons can be excited by absorbing energy from photons. Electrons can only be excited to certain electronic energy levels. Each electronic energy level is a number that represents the sum of the kinetic and potential energy (K+U). Because the electronic potential energy between the positive protons in the nucleus and the surrounding negative electrons will always be negative, the value of K+U will | ==The Main Ideas== | ||
===The Quantized Nature of Electronic Energy Levels=== | |||
Electrons can be excited by absorbing energy from photons. Electrons can only be excited to certain electronic energy levels. Each electronic energy level is a number that represents the sum of the kinetic and potential energy (K+U). Because the electronic potential energy between the positive protons in the nucleus and the surrounding negative electrons will always be negative, the value of K+U will be negative. Because electrons are only stable at those energy levels, an electron can only absorb certain quantized energies from photons. Once the electron absorbs a photon, it is excited by the energy. After the electron is excited, it drops down and releases a photon with the energy difference between the two energy levels. It can drop to any energy level below it, and thus the resulting photons can be of several energies. If the photon gained is the the opposite of the K+U value for the energy level, then the electron is said to have been ionized. The ionization energy of an atom is the energy needed to ionize an electron that is at rest. | |||
===The Nature of a Photon=== | ===The Nature of a Photon=== | ||
A photon falls neither in the category of a particle nor in the category of a wave. A photon behaves like a particle with a velocity, however it has no mass, and ceases to exist once its energy is absorbed. It can be created or destroyed at anytime, and thus cannot truly be considered as being a particle. It can be imagined as a elementary package of energy. It is a product of the wave-particle duality of light, which states that light behaves both as a particle was well as a wave. The relationship between the frequency of the wave of light and the energy contained in the photon can be described using Planck's Constant. | |||
====Photon as a Wave==== | |||
One demonstration of how photons act as waves is the double-slit experiment. When a beam of light/photons is shot at a panel with 2 slits in it, a striped pattern is formed on the other side. This is due to the wave-like interference between the two beams of light that are created by the two slits. Where the stripes themselves appear, the light waves are constructively interfering, or adding together. Between the stripes is darkness due to the destructive interference of the light waves. | |||
[[File:Two-Slit Experiment Light.svg|thumb|Double-Slit Experiment Light]] | |||
By measuring the distance y between the stripes of light, this phenomenon can also be used to determine the wavelength of the photons as long as the distance d between the slits is known as well as the distance x between the slitted panel and the wall. | |||
<math>\lambda = \frac{y*d}{x}</math> | |||
[[File:Untitled drawing.jpg|thumb|Double-Slit Experiment]] | |||
===Energy and Momentum=== | |||
Since photons move at the speed of light, their energy and momentum must be calculated relativistically. The momentum and energy of a photon can be calculated using the following equations, where <math>\lambda</math> is the wavelength (or the inverse of the frequency) and h is plank's constant (<math>6.62607015×10^{−34} J⋅Hz^{−1}</math>) | |||
<math>p=\frac{h}{\lambda}</math> | |||
<math>E=p*c=\frac{h*c}{\lambda}</math> | |||
===Uncertainty=== | |||
Due to the wave-particle duality of photons, they must obey the Heisenberg uncertainty principle, which states that the more accurately the location of the photon is defined, the less accurately the momentum can be defined. The reverse is true as well. | |||
<math>\Delta x \Delta p \approx \hbar \ge \frac{\hbar}{2}</math> | |||
In this equation, <math>\hbar</math> is the reduced plank's constant. | |||
<math>\hbar = \frac{h}{2\pi}</math> | |||
===A Mathematical Model of the Bohr Hydrogen Atom=== | ===A Mathematical Model of the Bohr Hydrogen Atom=== | ||
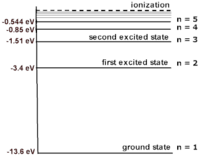
For example, the electronic energy levels for a hydrogen atom can be modeled by the equation: <math>{\frac{-13.6}{N^2}} = {K+U}</math> where '''N''' is the energy level. N=1 is the rest energy level; N=2 is the the first excited energy level; and N=3 is the second level, etc. This formula gives energy levels in terms of electron volts (eV). If you substitute values of N into the equation you can build the atom shown. As the value of N increases, the space between each energy level decreases. The energy difference between the rest energy level and the first excited energy level is the largest. Because the energy of the rest energy level is -13.6 eV, the ionization of an electron at rest in a hydrogen atom is 13.6 eV. In other words, if the electron at rest absorbs a photon with 13.6 eV, the electron is "freed" from the atom. In this | For example, the electronic energy levels for a hydrogen atom can be modeled by the equation: <math>{\frac{-13.6}{N^2}} = {K+U}</math> where '''N''' is the energy level. N=1 is the rest energy level; N=2 is the the first excited energy level; and N=3 is the second level, etc. This formula gives energy levels in terms of electron volts (eV). If you substitute values of N into the equation you can build the atom shown. As the value of N increases, the space between each energy level decreases. The energy difference between the rest energy level and the first excited energy level is the largest. Because the energy of the rest energy level is -13.6 eV, the ionization energy of an electron at rest in a hydrogen atom is 13.6 eV. In other words, if the electron at rest absorbs a photon with 13.6 eV, the electron is "freed" from the atom. In this atom, the difference between the the first (-13.6 eV) and second (-3.4 eV) energy level is 10.2 eV. This means that a photon needs to have a minimum energy of 10.2 eV to be absorbed by the electron and excite it. | ||
[[File:hydrogen.png|200px|thumb|left|Hydrogen and its energy levels]] | [[File:hydrogen.png|200px|thumb|left|Hydrogen and its energy levels]] | ||
===A Computational Model=== | ===A Computational Model of the Bohr Hydrogen Atom=== | ||
The following program is a computational model of the electron of a hydrogen atom absorbing a photon and jumping from the first energy level to the second. The speed of the photon (the yellow sphere) has been greatly scaled back for visual purposes. | |||
https://www.glowscript.org/#/user/zachshap88/folder/MyPrograms/program/idk | |||
The following program is a computational model of the electron of a hydrogen atom releasing a photon and jumping from the second energy level to the first. The speed of the photon (the yellow sphere) has been greatly scaled back for visual purposes. | |||
https://www.glowscript.org/#/user/zachshap88/folder/MyPrograms/program/photon2 | |||
==Examples== | ==Examples== | ||
===Photon Absorption=== | |||
If a hypothetical ion had the first 4 energy levels of -4, -2.3, -1.9, and -.8 eV, and an electron at rest was struck by a photon with 2.2 eV of energy, what is the highest excited state the electron could be at? How much energy would the photon leave with, if at all? | |||
First, check the amount of energy need to go from the rest energy level to the first excited energy level: -2.3-(-4)= 1.7. 2.2>1.7, so the electron will be excited to at least this state. If 1.7 eV is absorbed, then 2.2-1.7= .5 eV is remaining. Then check if this sufficient to raise it one more energy level. -1.9-(-2.3)= .4. .5 is greater than .4, so the photon also has sufficient energy to raise it to the second energy level. after raising it to the second excited level, the photon has .5-.4= .1 eV of energy remaining. This .1 eV is not sufficient to raise it to the third excited energy level. So, the photon will be at the second excited energy level, and the photon will have .1 eV of energy remaining. | |||
===Photon Emission=== | |||
If electrons of energy 12.8 eV are incident on a gas of hydrogen atoms in their ground state, what are the energies of the photons that are emitted by the excited gas? | |||
First, determine that the difference between the rest energy level (-13.6 eV) and the 3rd excited state (-.85 eV) IS 12.75 eV, and the remaining .05 eV is not sufficient to raise it another energy level. Then consider the different paths the electron could take back to its rest position, and calculate the energies of the corresponding photon emissions. Firstly, the electron could return one energy level at a time, releasing a a photon each drop. The differences between each energy level are: 10.2, 1.89, and .66 eV. Alternatively, the electron could drop from the the fourth energy level directly back to the first. This photon would have an energy equal to the difference between the 2 energy levels: 12.75 eV. Also, it could drop from 4th to 2nd to 1st, and the photon that would be emitted between the 4th and 2nd is 2.55. We have already accounted for the drop between the 2nd and 1st. Lastly, the electron could go from 4th to 3rd to 1st. The drop from 4th to 3rd has already been accounted for, and the difference between the 3rd and l is 12.09. In conclusion, all the possible energies for the emitted photons, from highest to lowest are: 12.75, 12.09, 10.2,2.55,1.89, and .66 eV. | |||
== | ==Historical Context== | ||
In the development of atomic theory, Rutherford discovered that atoms have a nucleus through his famous gold foil experiment. Then, Niels Bohr conjectured that electrons only travel in distinct energy levels around the nucleus. In 1913, Niels Bohr proposed a theory for the hydrogen atom based on quantum theory that energy is transferred only in certain well defined quantities. Electrons should move around the nucleus but only in prescribed orbits. When jumping from one orbit to another with lower energy, a light quantum is emitted. Bohr's theory could explain why atoms emitted light in fixed wavelengths. | |||
== See also == | == See also == | ||
[[Photons]], | |||
[[Bohr Model]], | |||
[[Electronic Energy Levels]] | |||
===External links=== | ===External links=== | ||
http://physics.about.com/od/quantumphysics/f/quantumoptics.htm | |||
http://dev.physicslab.org/document.aspx?doctype=3&filename=atomicnuclear_bohrmodelderivation.xml | |||
==References== | ==References== | ||
https://www.youtube.com/watch?v=Y0048AI5uEQ | |||
Matter and Interactions. 4th Edition. | |||
http://www.nobelprize.org/nobel_prizes/physics/laureates/1922/bohr-facts.html | |||
https://www.millersville.edu/physics/experiments/089/ | |||
https://courses.lumenlearning.com/austincc-physics2/chapter/27-3-youngs-double-slit-experiment/ | |||
[[Category: | [[Category:Energy]] | ||
Latest revision as of 23:02, 24 April 2022
Zachary Shapiro Spring 2022
In a classical Bohr model, electrons have discrete quantities of energy, called energy levels, as they orbit the central nucleus. An atom is thought to contain several "shells" in which electrons orbit the nucleus, and each shell represents a different energy level. Which shell an electron is currently in is denoted by the principal quantum number (n = 1,2,3...). Lower principal quantum numbers represent an electron shell that has a lower energy level and is closer to the nucleus. Electrons can transfer to a higher energy level by absorbing energy (usually in the form of a photon). However, the electron will eventually return to its ground state, releasing a photon in the process. Photons are the fundamental unit of electromagnetic radiation and are responsible for the phenomenon of light. They are considered to be massless and thus travel at the speed of light (299,792,458 m/s).
The Main Ideas
The Quantized Nature of Electronic Energy Levels
Electrons can be excited by absorbing energy from photons. Electrons can only be excited to certain electronic energy levels. Each electronic energy level is a number that represents the sum of the kinetic and potential energy (K+U). Because the electronic potential energy between the positive protons in the nucleus and the surrounding negative electrons will always be negative, the value of K+U will be negative. Because electrons are only stable at those energy levels, an electron can only absorb certain quantized energies from photons. Once the electron absorbs a photon, it is excited by the energy. After the electron is excited, it drops down and releases a photon with the energy difference between the two energy levels. It can drop to any energy level below it, and thus the resulting photons can be of several energies. If the photon gained is the the opposite of the K+U value for the energy level, then the electron is said to have been ionized. The ionization energy of an atom is the energy needed to ionize an electron that is at rest.
The Nature of a Photon
A photon falls neither in the category of a particle nor in the category of a wave. A photon behaves like a particle with a velocity, however it has no mass, and ceases to exist once its energy is absorbed. It can be created or destroyed at anytime, and thus cannot truly be considered as being a particle. It can be imagined as a elementary package of energy. It is a product of the wave-particle duality of light, which states that light behaves both as a particle was well as a wave. The relationship between the frequency of the wave of light and the energy contained in the photon can be described using Planck's Constant.
Photon as a Wave
One demonstration of how photons act as waves is the double-slit experiment. When a beam of light/photons is shot at a panel with 2 slits in it, a striped pattern is formed on the other side. This is due to the wave-like interference between the two beams of light that are created by the two slits. Where the stripes themselves appear, the light waves are constructively interfering, or adding together. Between the stripes is darkness due to the destructive interference of the light waves.

By measuring the distance y between the stripes of light, this phenomenon can also be used to determine the wavelength of the photons as long as the distance d between the slits is known as well as the distance x between the slitted panel and the wall.
[math]\displaystyle{ \lambda = \frac{y*d}{x} }[/math]
Energy and Momentum
Since photons move at the speed of light, their energy and momentum must be calculated relativistically. The momentum and energy of a photon can be calculated using the following equations, where [math]\displaystyle{ \lambda }[/math] is the wavelength (or the inverse of the frequency) and h is plank's constant ([math]\displaystyle{ 6.62607015×10^{−34} J⋅Hz^{−1} }[/math])
[math]\displaystyle{ p=\frac{h}{\lambda} }[/math]
[math]\displaystyle{ E=p*c=\frac{h*c}{\lambda} }[/math]
Uncertainty
Due to the wave-particle duality of photons, they must obey the Heisenberg uncertainty principle, which states that the more accurately the location of the photon is defined, the less accurately the momentum can be defined. The reverse is true as well.
[math]\displaystyle{ \Delta x \Delta p \approx \hbar \ge \frac{\hbar}{2} }[/math]
In this equation, [math]\displaystyle{ \hbar }[/math] is the reduced plank's constant.
[math]\displaystyle{ \hbar = \frac{h}{2\pi} }[/math]
A Mathematical Model of the Bohr Hydrogen Atom
For example, the electronic energy levels for a hydrogen atom can be modeled by the equation: [math]\displaystyle{ {\frac{-13.6}{N^2}} = {K+U} }[/math] where N is the energy level. N=1 is the rest energy level; N=2 is the the first excited energy level; and N=3 is the second level, etc. This formula gives energy levels in terms of electron volts (eV). If you substitute values of N into the equation you can build the atom shown. As the value of N increases, the space between each energy level decreases. The energy difference between the rest energy level and the first excited energy level is the largest. Because the energy of the rest energy level is -13.6 eV, the ionization energy of an electron at rest in a hydrogen atom is 13.6 eV. In other words, if the electron at rest absorbs a photon with 13.6 eV, the electron is "freed" from the atom. In this atom, the difference between the the first (-13.6 eV) and second (-3.4 eV) energy level is 10.2 eV. This means that a photon needs to have a minimum energy of 10.2 eV to be absorbed by the electron and excite it.
A Computational Model of the Bohr Hydrogen Atom
The following program is a computational model of the electron of a hydrogen atom absorbing a photon and jumping from the first energy level to the second. The speed of the photon (the yellow sphere) has been greatly scaled back for visual purposes. https://www.glowscript.org/#/user/zachshap88/folder/MyPrograms/program/idk
The following program is a computational model of the electron of a hydrogen atom releasing a photon and jumping from the second energy level to the first. The speed of the photon (the yellow sphere) has been greatly scaled back for visual purposes. https://www.glowscript.org/#/user/zachshap88/folder/MyPrograms/program/photon2
Examples
Photon Absorption
If a hypothetical ion had the first 4 energy levels of -4, -2.3, -1.9, and -.8 eV, and an electron at rest was struck by a photon with 2.2 eV of energy, what is the highest excited state the electron could be at? How much energy would the photon leave with, if at all?
First, check the amount of energy need to go from the rest energy level to the first excited energy level: -2.3-(-4)= 1.7. 2.2>1.7, so the electron will be excited to at least this state. If 1.7 eV is absorbed, then 2.2-1.7= .5 eV is remaining. Then check if this sufficient to raise it one more energy level. -1.9-(-2.3)= .4. .5 is greater than .4, so the photon also has sufficient energy to raise it to the second energy level. after raising it to the second excited level, the photon has .5-.4= .1 eV of energy remaining. This .1 eV is not sufficient to raise it to the third excited energy level. So, the photon will be at the second excited energy level, and the photon will have .1 eV of energy remaining.
Photon Emission
If electrons of energy 12.8 eV are incident on a gas of hydrogen atoms in their ground state, what are the energies of the photons that are emitted by the excited gas?
First, determine that the difference between the rest energy level (-13.6 eV) and the 3rd excited state (-.85 eV) IS 12.75 eV, and the remaining .05 eV is not sufficient to raise it another energy level. Then consider the different paths the electron could take back to its rest position, and calculate the energies of the corresponding photon emissions. Firstly, the electron could return one energy level at a time, releasing a a photon each drop. The differences between each energy level are: 10.2, 1.89, and .66 eV. Alternatively, the electron could drop from the the fourth energy level directly back to the first. This photon would have an energy equal to the difference between the 2 energy levels: 12.75 eV. Also, it could drop from 4th to 2nd to 1st, and the photon that would be emitted between the 4th and 2nd is 2.55. We have already accounted for the drop between the 2nd and 1st. Lastly, the electron could go from 4th to 3rd to 1st. The drop from 4th to 3rd has already been accounted for, and the difference between the 3rd and l is 12.09. In conclusion, all the possible energies for the emitted photons, from highest to lowest are: 12.75, 12.09, 10.2,2.55,1.89, and .66 eV.
Historical Context
In the development of atomic theory, Rutherford discovered that atoms have a nucleus through his famous gold foil experiment. Then, Niels Bohr conjectured that electrons only travel in distinct energy levels around the nucleus. In 1913, Niels Bohr proposed a theory for the hydrogen atom based on quantum theory that energy is transferred only in certain well defined quantities. Electrons should move around the nucleus but only in prescribed orbits. When jumping from one orbit to another with lower energy, a light quantum is emitted. Bohr's theory could explain why atoms emitted light in fixed wavelengths.
See also
Photons, Bohr Model, Electronic Energy Levels
External links
http://physics.about.com/od/quantumphysics/f/quantumoptics.htm http://dev.physicslab.org/document.aspx?doctype=3&filename=atomicnuclear_bohrmodelderivation.xml
References
https://www.youtube.com/watch?v=Y0048AI5uEQ
Matter and Interactions. 4th Edition.
http://www.nobelprize.org/nobel_prizes/physics/laureates/1922/bohr-facts.html
https://www.millersville.edu/physics/experiments/089/
https://courses.lumenlearning.com/austincc-physics2/chapter/27-3-youngs-double-slit-experiment/