Spontaneous Photon Emission: Difference between revisions
(Created page with "Spontaneous Photon Emission page in progress by kylerasmussen44 Photon Emission is a process that occurs when an atom or other quantum system goes down an energy level, and r...") |
No edit summary |
||
| Line 1: | Line 1: | ||
page in progress by kylerasmussen44 | page in progress by kylerasmussen44 | ||
Spontaneous photon emission is a process that occurs when an atom or other quantum system goes down an energy level, and releases a photon. This process is often incited by the absorption of a particle whose energy causes an atom to increase its energy level, and enter an excited state; in this case, spontaneous photon emission would move the atom to a lower energy level, closer to its initial state. | |||
==The Main Idea== | |||
If an atom is in an excited state, meaning that its current energy level is higher than the minimum energy level, or ground state, it may undergo the process of spontaneous photon emission, decreasing its energy level to one closer to the ground state. Through this process, an atom will decrease its energy level, and emit a photon with energy equal to the difference in energy between the two energy levels. | |||
[[File:Spontaneousemission.png]] | |||
If we chose a system including both the photon and the atom, this process will feature no net energy change. | |||
A Computational Model | ===A Mathematical Model=== | ||
How do we visualize or predict using this topic. Consider embedding some vpython code here Teach hands-on with GlowScript | |||
What are the mathematical equations that allow us to model this topic. For example <math>{\frac{d\vec{p}}{dt}}_{system} = \vec{F}_{net}</math> where '''p''' is the momentum of the system and '''F''' is the net force from the surroundings. | |||
===A Computational Model=== | |||
How do we visualize or predict using this topic. Consider embedding some vpython code here [https://trinket.io/glowscript/31d0f9ad9e Teach hands-on with GlowScript] | |||
==Examples== | |||
Be sure to show all steps in your solution and include diagrams whenever possible | Be sure to show all steps in your solution and include diagrams whenever possible | ||
Simple | ===Simple=== | ||
Middling | ===Middling=== | ||
Difficult | ===Difficult=== | ||
Connectedness | |||
How is this topic connected to something that you are interested in? | ==Connectedness== | ||
How is it connected to your major? | #How is this topic connected to something that you are interested in? | ||
Is there an interesting industrial application? | #How is it connected to your major? | ||
History | #Is there an interesting industrial application? | ||
==History== | |||
Put this idea in historical context. Give the reader the Who, What, When, Where, and Why. | Put this idea in historical context. Give the reader the Who, What, When, Where, and Why. | ||
See also | == See also == | ||
Are there related topics or categories in this wiki resource for the curious reader to explore? How does this topic fit into that context? | |||
Are there related topics or categories in this wiki resource for the curious reader to explore? How does this topic fit into that context? | |||
===Further reading=== | |||
Books, Articles or other print media on this topic | Books, Articles or other print media on this topic | ||
External links[ | ===External links=== | ||
[http://www.scientificamerican.com/article/bring-science-home-reaction-time/] | |||
==References== | |||
This section contains the the references you used while writing this page | This section contains the the references you used while writing this page | ||
Category: Which Category did you place this in? | [[Category:Which Category did you place this in?]] | ||
Revision as of 21:46, 3 December 2015
page in progress by kylerasmussen44
Spontaneous photon emission is a process that occurs when an atom or other quantum system goes down an energy level, and releases a photon. This process is often incited by the absorption of a particle whose energy causes an atom to increase its energy level, and enter an excited state; in this case, spontaneous photon emission would move the atom to a lower energy level, closer to its initial state.
The Main Idea
If an atom is in an excited state, meaning that its current energy level is higher than the minimum energy level, or ground state, it may undergo the process of spontaneous photon emission, decreasing its energy level to one closer to the ground state. Through this process, an atom will decrease its energy level, and emit a photon with energy equal to the difference in energy between the two energy levels.
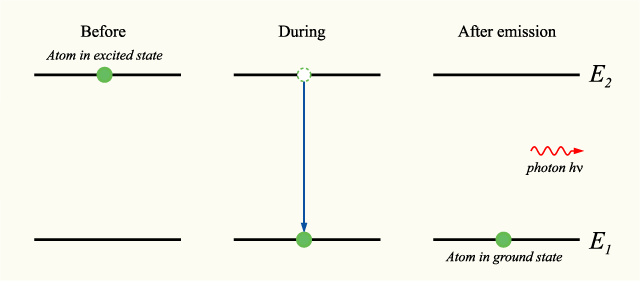 If we chose a system including both the photon and the atom, this process will feature no net energy change.
If we chose a system including both the photon and the atom, this process will feature no net energy change.
A Mathematical Model
What are the mathematical equations that allow us to model this topic. For example [math]\displaystyle{ {\frac{d\vec{p}}{dt}}_{system} = \vec{F}_{net} }[/math] where p is the momentum of the system and F is the net force from the surroundings.
A Computational Model
How do we visualize or predict using this topic. Consider embedding some vpython code here Teach hands-on with GlowScript
Examples
Be sure to show all steps in your solution and include diagrams whenever possible
Simple
Middling
Difficult
Connectedness
- How is this topic connected to something that you are interested in?
- How is it connected to your major?
- Is there an interesting industrial application?
History
Put this idea in historical context. Give the reader the Who, What, When, Where, and Why.
See also
Are there related topics or categories in this wiki resource for the curious reader to explore? How does this topic fit into that context?
Further reading
Books, Articles or other print media on this topic
External links
References
This section contains the the references you used while writing this page