Electromagnetic Spectrum: Difference between revisions
Croberts65 (talk | contribs) No edit summary |
Croberts65 (talk | contribs) No edit summary |
||
| Line 34: | Line 34: | ||
===Middling=== | ===Middling=== | ||
===Difficult=== | ===Difficult=== | ||
==History== | |||
The first time that a part of the electromagnetic spectrum was observed was in 1800, when scientist William Herschel was looking at light from a heated object through a prism. He noticed that the light surpassed the color red, into what we now know today as the infrared frequency of light. Some years later in 1845, Micheal Faraday made the connection between light and electromagnetism, when he observed that polarized light responded to a magnet. James Maxwell made the final step, when he realized that electromagnetic waves must travel at the speed of light. | |||
==Connectedness== | ==Connectedness== | ||
| Line 39: | Line 43: | ||
#How is it connected to your major? | #How is it connected to your major? | ||
#Is there an interesting industrial application? | #Is there an interesting industrial application? | ||
==References & Further Reading== | ==References & Further Reading== | ||
Revision as of 00:00, 4 December 2015
This page has been created and claimed by Clayton Roberts (Croberts65)
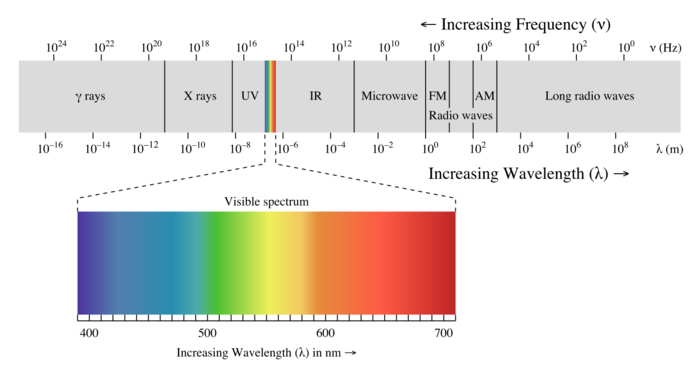
The electromagnetic spectrum describes all different frequencies of light that can be observed, and is most commonly associated with the visible chromatic colors.
The Main Idea
The frequency in Hertz (Hz) of electromagnetic waves can be related to the energy and color (if applicable) of the type of electromagnetic radiation. This can be used in chemistry for ionization, among other fields of science where the material and relative temperature can be related.
A Mathematical Model
[math]\displaystyle{ v = {\frac{c}{\lambda}} }[/math] where
- [math]\displaystyle{ v }[/math] is the frequency of the electromagnetic wave in Hertz (Hz) or number of cycles a second and can also be written as "[math]\displaystyle{ f }[/math]"
- [math]\displaystyle{ c }[/math] is the speed of light ( [math]\displaystyle{ 2.998 \times 10^8 \frac{m}{s} }[/math] )
- [math]\displaystyle{ \lambda }[/math] is the wavelength in meters
[math]\displaystyle{ E = \sqrt {\frac{energy}{c \times \epsilon_{0}}} }[/math] where
- [math]\displaystyle{ E }[/math] is the magnitude of the electric field in Newtons per Columb (N/C)
- [math]\displaystyle{ \epsilon_{0} }[/math] is the vacuum permittivity constant ( [math]\displaystyle{ 8.85 \times 10^{-12} }[/math] )
- [math]\displaystyle{ c }[/math] is the speed of light ( [math]\displaystyle{ 2.998 \times 10^8 \frac{m}{s} }[/math] )
A Visual Model
How do we visualize or predict using this topic. Consider embedding some vpython code here Teach hands-on with GlowScript
Examples
Be sure to show all steps in your solution and include diagrams whenever possible
Simple
Middling
Difficult
History
The first time that a part of the electromagnetic spectrum was observed was in 1800, when scientist William Herschel was looking at light from a heated object through a prism. He noticed that the light surpassed the color red, into what we now know today as the infrared frequency of light. Some years later in 1845, Micheal Faraday made the connection between light and electromagnetism, when he observed that polarized light responded to a magnet. James Maxwell made the final step, when he realized that electromagnetic waves must travel at the speed of light.
Connectedness
- How is this topic connected to something that you are interested in?
- How is it connected to your major?
- Is there an interesting industrial application?
References & Further Reading
[Electromagnetic Spectrum] [Vacuum Permittivity] [Electric Field]