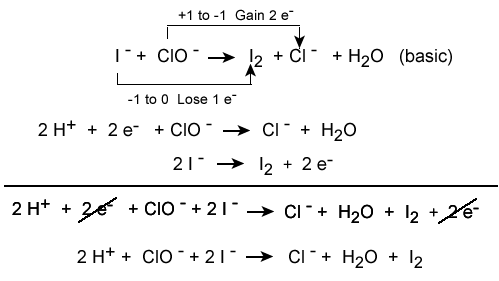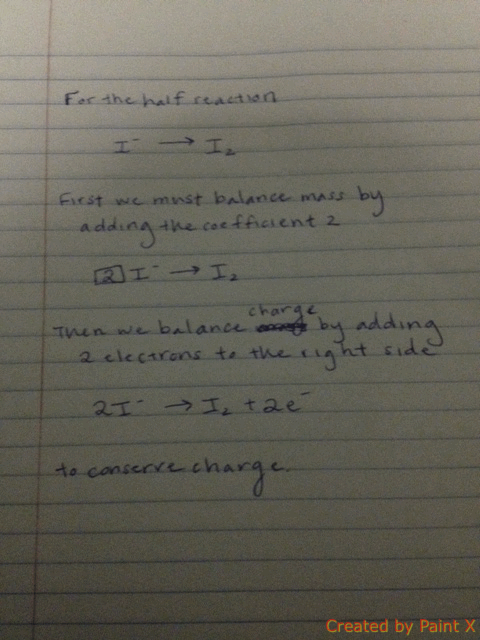Conservation of Charge: Difference between revisions
| Line 47: | Line 47: | ||
Thorium-234 decays by emitting a beta particle (charge of -1). What is the number of electrons after the decay? | Thorium-234 decays by emitting a beta particle (charge of -1). What is the number of electrons after the decay? | ||
Solution: | Solution: | ||
[[File:Thoriumdecay.gif]] | |||
==Connectedness== | ==Connectedness== | ||
Revision as of 18:47, 5 December 2015
oduan3
Conservation of charge is the principle that the sum of the electrical charge of a closed system is constant.
The Main Idea
The idea that the net charge of a closed system is constant implies that: if a charge appears in a previously neutral system, an equal and opposite charge appears in another part of the system. Individual charges, however, can be created or destroyed. The sum of all electrical charge in the universe, then, is assumed to be some constant quantity (presumably zero, although this is not certain).
A Mathematical Model
Given the intial and final times (Ti and Tf), the charge of a system can be described using the equation Q(Tf) = Q(Ti) + Qin - Qout. This allows us to predict the charge of a system after a given time has passed by adding the total flow of charge into the system and subtracting the total flow of charge out of the system.
Furthermore, conservation of charge is described by one of Maxwell's equations
which states that the divergence of current density (J) is proportional to the change in charge density (rho).
A Computational Model
How do we visualize or predict using this topic. Consider embedding some vpython code here Teach hands-on with GlowScript
Examples
Simple
A system contains three particles: particle 1 has a charge of 2Q, particle 2 has a charge of -2Q, and particle 3 has a charge of 3Q. What is the net charge on the system?
Solution:
Add the charges together to get the net charge.
-2 + 2 + 3 = 3 Q
Middling
A system contains two spheres of the same mass. Sphere 1 has a charge of -1C and Sphere 2 has a charge of + 5C. The two spheres are brought together, touch, and are separated again. What is the net charge on each of the two spheres?
Solution:
Since the two spheres have the same mass, the charge will distribute itself evenly between the two spheres. Add the two charges, then divide by 2.
(-1+5)/2 = 3 C
Difficult
Thorium-234 decays by emitting a beta particle (charge of -1). What is the number of electrons after the decay?
Solution:
Connectedness
Conservation of charge has practical applications in circuits, especially as it relates to Kirchhoff's rules. The junction rule states that the amount of current (charge per time) flowing into a junction is equal to the current flowing out of a junction.
Conservation of charge is relevant to chemistry, especially for predicting the outcome of reactions (redox reactions in particular). For example, the typical sodium and chlorine ions have equal and opposite charges, and when they react, they form a neutral compound via the transfer of one electron to another, which abides by the conservation of charge rule (-1 + 1 = 0). The process of balancing chemical equations requires that the net charge on each side of the equation be equal, so electrons are "added" to each side as necessary to balance out the charges.
Balancing each half-reaction requires knowledge of conservation of mass and conservation of charge, as demonstrated below.
History
Put this idea in historical context. Give the reader the Who, What, When, Where, and Why.
See also
Are there related topics or categories in this wiki resource for the curious reader to explore? How does this topic fit into that context?
Further reading
Books, Articles or other print media on this topic
External links
References
This section contains the the references you used while writing this page