Spontaneous Photon Emission: Difference between revisions
No edit summary |
No edit summary |
||
| Line 1: | Line 1: | ||
Page written by Kyle Rasmussen | |||
Spontaneous photon emission is a process that occurs when an atom or other quantum system goes down an energy level, and releases a photon. This process is often incited by the absorption of a particle whose energy causes an atom to increase its energy level, and enter an excited state; in this case, spontaneous photon emission would move the atom to a lower energy level, closer to its initial state. This process results in the production of light, and has been instrumental in many inventions, such as fluorescent lights, television displays and light emitting diodes. | Spontaneous photon emission is a process that occurs when an atom or other quantum system goes down an energy level, and releases a photon. This process is often incited by the absorption of a particle whose energy causes an atom to increase its energy level, and enter an excited state; in this case, spontaneous photon emission would move the atom to a lower energy level, closer to its initial state. This process results in the production of light, and has been instrumental in many inventions, such as fluorescent lights, television displays and light emitting diodes. | ||
Revision as of 18:14, 5 December 2015
Page written by Kyle Rasmussen
Spontaneous photon emission is a process that occurs when an atom or other quantum system goes down an energy level, and releases a photon. This process is often incited by the absorption of a particle whose energy causes an atom to increase its energy level, and enter an excited state; in this case, spontaneous photon emission would move the atom to a lower energy level, closer to its initial state. This process results in the production of light, and has been instrumental in many inventions, such as fluorescent lights, television displays and light emitting diodes.
The Main Idea
If an atom is in an excited state, meaning that its current energy level is higher than the minimum energy level, or ground state, it may undergo the process of spontaneous photon emission, decreasing its energy level to one closer to the ground state. Through this process, an atom will decrease its energy level, and emit a photon with energy equal to the difference in energy between the two energy levels.
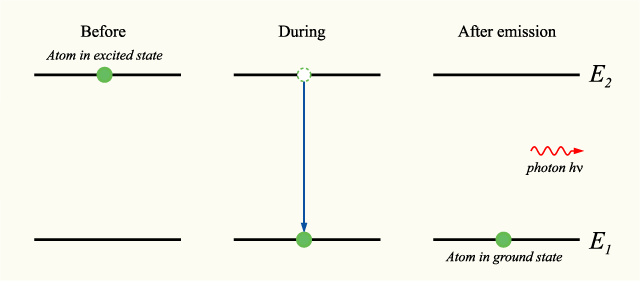 In accordance with the law of conservation of energy, if we chose a system including both the photon and the atom, this process will feature no net energy change.
The collection of photon emissions for an atom's transition from a higher to a lower state is called an emission spectrum. For any given atom in an excited state, there typically exists a wide range of potential photon emissions, and these emissions vary greatly between different elements.
In accordance with the law of conservation of energy, if we chose a system including both the photon and the atom, this process will feature no net energy change.
The collection of photon emissions for an atom's transition from a higher to a lower state is called an emission spectrum. For any given atom in an excited state, there typically exists a wide range of potential photon emissions, and these emissions vary greatly between different elements.


Above you'll see the emissions spectrums for hydrogen and krypton respectively. As you can see, krypton has a much wider range of potential photon emissions, largely because its atoms are far more complex than those of a more simple element like hydrogen.
Mathematical Model
The primary basis for our understanding of the process of spontaneous photon emission comes from the law of conservation of energy, [math]\displaystyle{ {E}_{final} = {E}_{initial} }[/math].
When an atom decreases its energy level, as they do during spontaneous photon emission, this energy cannot be lost, in accordance with the law of conservation of energy. Instead, it is simply converted into another form, in this case, the kinetic energy of a photon. Because this process only involves two different particles, the atom and the photon, the law of conservation of energy also allows us to know that the change in the energy of the atom, is equal and opposite the change in the energy of the photon, [math]\displaystyle{ {Δ}{E}_{atom} = {-}{Δ}{E}_{photon} }[/math].
A Computational Model
How do we visualize or predict using this topic. Consider embedding some vpython code here Teach hands-on with GlowScript
Examples
Be sure to show all steps in your solution and include diagrams whenever possible
Simple
The energy of an atom decreases from -2 to -8 eV. A photon is emitted during this process, what is the energy of this photon?
[math]\displaystyle{ {E}_{photon} = {-}{Δ}{E}_{atom} = {-}{(}{E}_{f} - {E}_{i}{)} = {-}{(}{-8} - {-2}{)} = {6}{eV} }[/math]
Middling
A hydrogen atom is in the state N=3, where N=1 is the ground state. What will be the energy of the photon emitted when this atom drops from the 3rd to 1st energy level?
[math]\displaystyle{ {E}_{3} = {-}{13.6}/{3^2} = {-}{1.5111}{eV} }[/math]
[math]\displaystyle{ {E}_{1} = {-}{13.6}/{1} = {-}{13.6}{eV} }[/math]
[math]\displaystyle{ {E}_{photon} = {-}{Δ}{E}_{atom} = {-}{(}{E}_{1} - {E}_{3}{)} = {-}{(}{13.6} - {1.5111}{)} = {12.0889}{eV} }[/math]
Difficult
If electrons with energies of 10.27 volts collide with hydrogen atoms in their ground state, what will be the energy of the photons emited by these atoms?
[math]\displaystyle{ {E}_{1} = {-}{13.6}/{1} = {-}{13.6}{eV} }[/math]
[math]\displaystyle{ {E}_{2} = {-}{13.6}/{2^2} = {-}{3.4}{eV} }[/math]
[math]\displaystyle{ {E}_{photon} = {-}{Δ}{E}_{atom} = {-}{(}{E}_{1} - {E}_{2}{)} = {-}{(}{13.6} - {3.4}{)} = {10.2}{eV} }[/math]
Because 10.2 < 10.27, the electrons will be able to excite the atoms to this energy level, and photons of energy 10.2 eV will be released when these atoms return to their ground state.
What will be the energy of the electrons after the collision?
Because [math]\displaystyle{ {Δ}{E}_{total} = {0} }[/math]
[math]\displaystyle{ {E}_{electron final} = {E}_{electron initial} - {Δ}{E}_{atom} = {10.27} - {10.2} = {.07}{eV} }[/math]
Connectedness
The primary use of spontaneous photon emission in industry is in the creation of products that center around the emission of light, such as fluorescent lights, light emitting diodes, and plasma TV screens. This process allows for the production of photons through the energizing of atoms, and can be extremely helpful for creating controlled light sources.
History
The concept of spontaneous emission was first theorized and observed by Albert Einstein, who accurately predicted that an atom may return to a lower energy level by emitting a photon. Einstein also proposed the concept that photons would tend to travel together in the same state. He theorized that a photon with a certain wavelength could pass through a field of atoms, leading to the emission of a photon with that same wavelength by the atoms. He predicted that this would lead to a ripple effect throughout this collection of atoms, that would lead to the production of photons of this same wavelength by all the surrounding atoms. This concept was first addressed in a paper written by Einstein in 1917, but was not put to use fully until the 1940s, when Charles Townes and Arthur Schawlow developed the first functioning laser.
See also
Quantized Energy Levels: http://www.physicsbook.gatech.edu/Quantized_energy_levels
External links
References
"Emission Spectrum." Wikipedia. Wikimedia Foundation, 29 Nov. 2015. Web. 05 Dec. 2015.
"This Month in Physics History." : Einstein Predicts Stimulated Emission. American Physical Society, Aug.-Sept. 2005. Web. 05 Dec. 2015.
"Spontaneous Emission." Encyclopedia of Laser Physics and Technology. RP Photonics Encyclopedia, n.d. Web. 05 Dec. 2015. <https://www.rp-photonics.com/spontaneous_emission.html>.
"Spontaneous Emission." Spontaneous Emission. The University of Texas as Austin, n.d. Web. 05 Dec. 2015. <http://farside.ph.utexas.edu/teaching/qmech/Quantum/node119.html>.
"Spontaneous Emission." Wikipedia. Wikimedia Foundation, 4 Sept. 2015. Web. 05 Dec. 2015.