Temperature: Difference between revisions
No edit summary |
Ayeshaahuja (talk | contribs) No edit summary |
||
| Line 1: | Line 1: | ||
Written by Ju Yup Kim | Written by Ju Yup Kim | ||
'''Edited/Claimed by Ayesha Ahuja''' | |||
==Defining temperature== | ==Defining temperature== | ||
Revision as of 16:19, 23 November 2016
Written by Ju Yup Kim Edited/Claimed by Ayesha Ahuja
Defining temperature
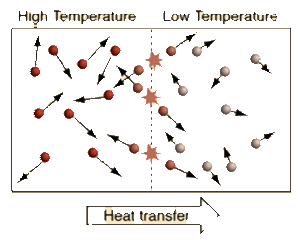
Temperature is measure of average kinetic energy of the particles in a system. Difference between temperature and heat is that heat is the sum of all the kinetic energies of the particles in a system. Adding heat to a system causes its temperature to rise. Newton's zeroth law states that a system reaches a thermal equilibrium when there is no observable change in temperature between a system. Therefore, the change in temperature causes heat to flow from a high temperature system to a low temperature system. When systems with different temperature is in contact, molecules with higher kinetic energy collide with molecules with lower kinetic energy, kinetic energy is passed from the molecules with more kinetic energy to those with less kinetic energy. This molecular level of kinetic energy transfer will happen until average kinetic energy of the particles in each systems reach the average of two.
The relationship between heat transfer and temperature can be modeled with this equation: Q = m c Delta T
Measurement
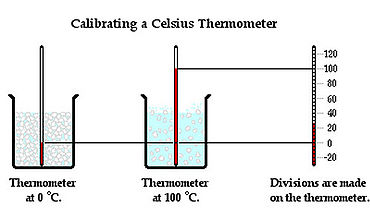
A thermometer is a device used to measure temperature. It is placed in contact with an object and allowed to reach thermal equilibrium with the object. The operation of a thermometer is based on some property, such as volume, that varies with temperature. For example, mercury in a mercury thermometer expands to a degree depending on a temperature of the object and the level of the mercury in side the glass tube rises or descends. Temperature can be measured in numbers by three temperature scales: Celsius, Fahrenheit, and Kelvin. Celsius scale sets freezing point of the water at zero and boiling point at 100, and Fahrenheit scale sets freezing point of the water at 32 degrees and boiling point of the water at 212 degrees. Kelvin scale is designed to go to zero at absolute zero, the minimum temperature.
The relationship between temperature scales: (degrees)K = 273.15 + (degrees)C (degrees)C = (5/9)*((degrees)F-32) (degrees)F = (9/5)*(degrees)C+32
Thermal Expansion
Change in temperature through heat transfer can change the matter to change in shape, area, and volume. When a substance is heated, the kinetic energy of its molecules increases. Then the molecules begin moving more and average separation between molecules become larger, and thus volume of the substance changes. The degree of expansion divided by the change in temperature is called the material's coefficient of thermal expansion and generally varies with temperature and the object and state of matter of the object.
| Material | Fractional expansion per degree C x10^-6 | Fractional expansion per degree F x10^-6 |
|---|---|---|
| Glass, ordinary | 9 | 5 |
| Glass, pyrex | 4 | 2.2 |
| Quartz, fused | .59 | .33 |
| Aluminum | 24 | 13 |
| Brass | 19 | 11 |
| Copper | 17 | 9.4 |
| Iron | 12 | 6.7 |
| Steel | 13 | 7.2 |
| Platinum | 9 | 5 |
| Tungsten | 4.3 | 2.4 |
| Gold | 14 | 7.8 |
| Silver | 18 | 10 |
History
Early explanations of heat were thoroughly confused with explanations of combustion. J. J. Becher and George Ernst Stahl introduced the phlogiston theory of combustion in the 17th century, and phlogiston was thought to be the substance of heat.
In late 18th century, Antoine Lavoisier argued that phlogiston theory is inconsistent and introduced Caloric theory. The suggested explanation was that when an object was heated, an invisible fluid called “caloric” was added to the object. Hot objects contained more caloric than cold objects. Caloric theory was popularly accepted until mid 19th century.
Followed by introduction of caloric theory, Count Rumford found that boring a cannon repeatedly does not result in heat producing ability, and therefore caloric is not lost. This proposed that caloric might not be a substance though the experimental ambiguity in his experiment were widely considered controversial.
In mid 19th century, Rudolf Clausius showed that Rumford's argument and Caloric theory can be agreeable if the caloric theory include the principle of conservation of energy in place of its original principle of conservation of heat. By doing so, the caloric theory was able to be evolved into modern thermodynamics where heat may be put as a thermal energy which is equivalent to kinetic energy of some particles of the substance.
References
http://hyperphysics.phy-astr.gsu.edu/hbase/tables/thexp.html
http://hyperphysics.phy-astr.gsu.edu/hbase/thermo/temper.html