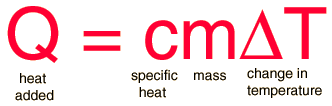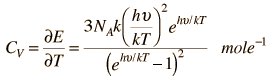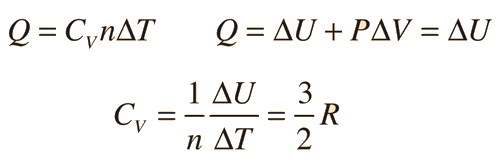Specific Heat: Difference between revisions
No edit summary |
|||
| Line 101: | Line 101: | ||
There are two specific heats for gases., one for gases at a constant volume and one gases at a constant pressure. For a constant volume process with a monoatomic ideal gas the first law of thermodynamics gives | There are two specific heats for gases., one for gases at a constant volume and one gases at a constant pressure. For a constant volume process with a monoatomic ideal gas the first law of thermodynamics gives: | ||
[[File:Specific Heat of Gas.gif]] | [[File:Specific Heat of Gas.gif]] | ||
where Cv is specific heat, n is number of moles, delta U is change in potential energy, delta T is change in temperature, and P is pressure. | |||
===Simple=== | ===Simple=== | ||
Revision as of 22:26, 5 December 2015
Short Description of Topic
The Main Idea
The most common definition is that specific heat is the amount of heat needed to raise the temperature of a mass by 1 degree. The relationship between heat and temperature change is best defined by constant "C" in the equation
The relationship does not apply if a phase change is occurs because the heat added or removed during a phase change does not necessarily change the temperature. The specific heat most commonly known specific heat is 4.16 J/g degrees Celsius, which is the specific heat for water. The specific heat per gram for water is much higher than that for a metal. Therefore, there are two separate ways to calculate specific heats. Traditionally, it is more acceptable to compare specific heats on a molecular level.
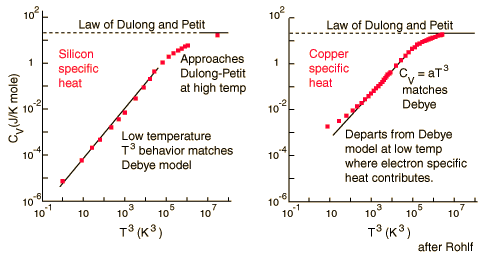
The molar specific heats of most solids at room temperature are almost the same, which agrees with the Law of Dulong and Petit. At lower temperatures the specific heats drop as atomic processes become more relevant. The lower temperature behavior is explained by the Einstein-Debye model of specific heat.
Law of Dulong and Petit
[edit]
The specific heat of copper is 0.386 Joules/gram degrees Celsius while the specific heat of Aluminum is 0.900 Joules/gram Celsius. Why is there such a difference? Specific heat is measured in Energy per unit mass, but it should be measured in Energy per mole for more similar specific heats for solids. The similar molar specific heats for solid metals are what define the Law of Dulong and Petit.
The specific heats of metals, therefore should all be around 24.94 J/mol degrees Celsius. The specific heat at constant volume should be just the temperature derivative of that energy.
Copper 0.386 J/gm K x 63.6 gm/mol = 24.6 J/mol K
Aluminum 0.900 J/gm K x 26.98 gm/ mol = 24.3 J/mol K
Einstein Debye Model
[edit]
For low temperatures, Einstein and Debye found that the Law of Dulong and Petit was not applicable. At lower temperatures, it was found that atomic interactions were deemed significant in calculating the molar specific heat of an object.
According to the Einstein Debye Model for Copper and Aluminum, two solid metals, specific heat varies much at lower temperatures and goes much below the Dulong-Petit Model. This is due to increased effects on specific heat by interatomic forces. However, for very high temperature values, the Einstein-Debye Model cannot be used. In fact, at high temperatures, Einstein's expression of specific heat, reduces to the Dulong-Petit mathematical expression.
Here is the Einstein Debye Equation:
For high Temperatures it may be reduced like this:
This actually reduces to the Dulong-Petit Formula for Specific Heat:
Specific Heats of Gases
Specific heats of gases are generally expressed in their molar form due to the undefined volume or pressure of a gas. Usually only one is held constant.
There are two specific heats for gases., one for gases at a constant volume and one gases at a constant pressure. For a constant volume process with a monoatomic ideal gas the first law of thermodynamics gives:
where Cv is specific heat, n is number of moles, delta U is change in potential energy, delta T is change in temperature, and P is pressure.
Simple
Middling
Difficult
Connectedness
- How is this topic connected to something that you are interested in?
- How is it connected to your major?
- Is there an interesting industrial application?
History
Put this idea in historical context. Give the reader the Who, What, When, Where, and Why.
See also
Are there related topics or categories in this wiki resource for the curious reader to explore? How does this topic fit into that context?
Further reading
Books, Articles or other print media on this topic
External links
References
This section contains the the references you used while writing this page
Template
Claimed by Felix Joseph
Main page
Recent changes
Random page
Help
Tools
What links here Related changes Upload file Special pages Printable version Permanent link Page information
This page was last modified on 29 November 2015, at 23:04. This page has been accessed 525 times. Privacy policy About Physics Book Disclaimers