Nuclear Fission: Difference between revisions
| Line 18: | Line 18: | ||
Nuclear power plants like these are found in various countries all over the globe. They work by heating water to boiling with nuclear fission. The water turns to steam and turns turbines, generating electricity. | Nuclear power plants like these are found in various countries all over the globe. They work by heating water to boiling with nuclear fission. The water turns to steam and turns turbines, generating electricity. | ||
[[File:NuclearPowerPlant2.png]] | [[File:NuclearPowerPlant2.png]] | ||
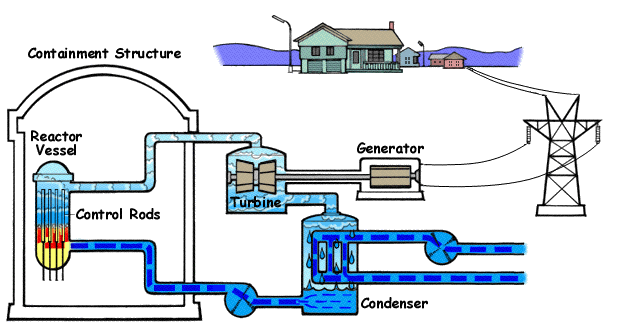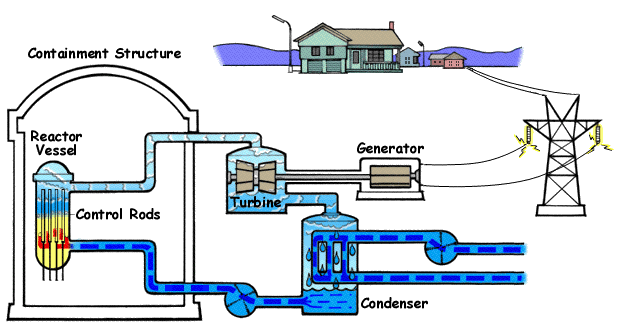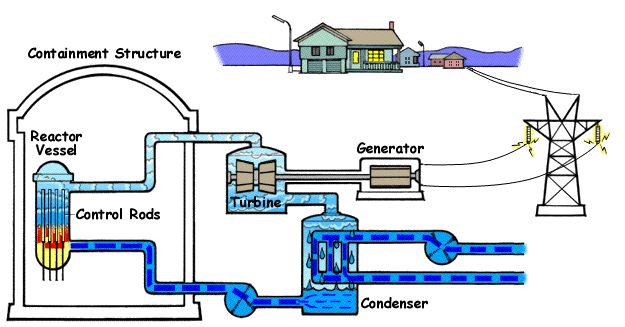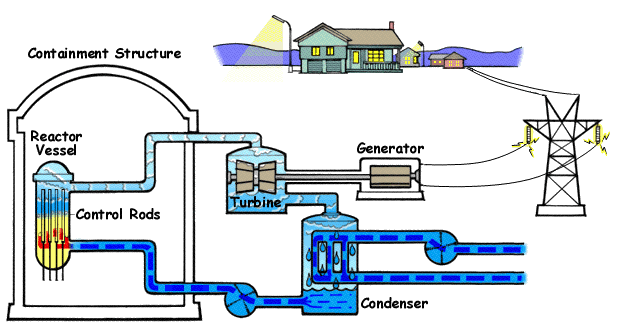
Below is a diagram illustrating | Below is a diagram illustrating a BWR(Boiling Water Reactor) and how they generate electricity with nuclear fission and water. | ||
[[File:BoilingWaterReactor.gif]] | [[File:BoilingWaterReactor.gif]] | ||
Revision as of 14:08, 9 April 2017
This topic is claimed by qmurphy3 NO3 Schatz.
Nuclear fission is the process of splitting up an atom into multiple parts. This occurs spontaneously in the form of radioactive decay.
The Main Idea
Nuclear fission is the process of the nucleus of an atom splitting into multiple smaller parts and, in doing so, releasing a quantity of energy. This process can happen naturally in the form of radioactive decay or in a nuclear reaction. Nuclear fission by radioactive decay is a natural process whereby a nucleus that is very big and unstable spontaneously breaks apart into two or three smaller nuclei and emitting particles such as neurons and gamma radiation in the process. Radioactive decay is very uncommon amongst most large molecules but does happen naturally for Uranium-235 and Plutonium-239, both of which are isotopes. Uranium-235 fissions when gains extra neurons that trigger its decay. The two substituents that form from the split atom have a mass that is about one tenth of one percent less mass than that of the original atom. Nuclear fission by nuclear reaction occurs when one nucleus collides with either another nucleus or a particle causing the nucleus to break apart into other smaller nuclei and releasing particles and gamma radiation. This can cause chain reactions whereby particles emitted when one nucleus fissions go on to collide with other nuclei and cause them to fission which releases more particles that go on to collide with even more nuclei. Nuclear fission reactions are typically managed to produce a standard and controlled reaction (such as the process to produce electricity) but when it is not managed it results in a dangerous and uncontrollable release of energy (such as the atomic bomb).
A Mathematical Model
The large amount of energy that is released is not lost or destroyed according to the conservation of mass principle, therefore we can use the formula below to better understand the change in mass and energy released during nuclear fission reactions.
Mass of energy released = (E/c^2) = Mass Final- Mass Initial
A Computational Model
Examples
Nuclear power plants like these are found in various countries all over the globe. They work by heating water to boiling with nuclear fission. The water turns to steam and turns turbines, generating electricity.
 Below is a diagram illustrating a BWR(Boiling Water Reactor) and how they generate electricity with nuclear fission and water.
Below is a diagram illustrating a BWR(Boiling Water Reactor) and how they generate electricity with nuclear fission and water.
Connectedness
Nuclear fission is a very interesting topic to me because of its potential for green and renewable energy. After delving deeper into the subject, I was made aware of just how astonishingly large the amount of energy that is produced from the splitting of an atom actually was. I was always aware of what nuclear fission was because of its use in the atomic bomb and the basic explanation in secondary school. This topic however does not directly tie into my major of Biomedical engineering. The results of effective nuclear fission would be energy with which to power some types of biomedical devices as well as the potential to learn more about elements and materials that could be used in nuclear fission and other things. The overall industrial application of nuclear fission is actually quite impressive, it has the potential to be one of the most reliable and consistent forms of power production once the remainder of fossil fuels are depleted. Nuclear fission is a growing form of producing energy and will become an even better alternative once there is an adequate way to dispose of the radioactive waste that it produces.
History
In 1896, French physicist Henri Becquerel noticed that uranium fluoresces under UV light and conducts experiments to examine this phenomena. Among the most famous of these experiments is one involving a clear piece of photographic film wrapped in black paper. A piece of uranium is placed on top of the wrapped film and left in the sunlight. When unwrapped, Becquerel finds that the clear film has become foggy. He believed that the uranium absorbs the sunlight and releases invisible ray that penetrate the paper and dye the film. However, upon further investigation, Becquerel is baffled to find that the uranium dyes the film even when placed in the drawer without any light. This must mean that some form of energy comes from inside the uranium. Ernest Rutherford and Frederick Soddy take to studying uranium on a quantum level. They discover that this element has a large nucleus that is so unstable, it spits out small pieces of itself containing protons, thereby becoming a completely different element. They watch uranium transform into Thorium. The thorium, also radioactive becomes Protactinium. This continues for fourteen different elements, the last of which being lead. A student of Rutherford, Otto Hahn, experiments with forcing uranium to change. Hahn and his assistant discover that it is actually possible to split the nucleus of the uranium atom. Together they discover that only uranium-235 will split. With 235 protons and neutrons, the nucleus is so huge and under so much strain, adding just one neutron makes the nucleus so unstable it causes the nucleus to fission. Hahn won the Nobel Prize in 1945 for discovering chemical proof of nuclear fission.
See also
Nuclear Fission and Radioactive Decay History of Nuclear Energy Nuclear Energy from Fission and Fusion
Further reading
"The Nuclear Fission Process" by Cyriel Wagemans "Nuclear Fission" by Robert Vandenbosch
References
Chabay, Ruth W., and Bruce A. Sherwood. "Chapter 10: Collisions." Matter & Interactions. Fourth Edition ed. Wiley, 2015. 261. Print. http://hyperphysics.phy-astr.gsu.edu/hbase/nucene/fission.html http://ieer.org/resource/factsheets/basics-nuclear-physics-fission/ https://en.wikipedia.org/wiki/Nuclear_fission https://en.wikipedia.org/wiki/Otto_Hahn http://chemed.chem.purdue.edu/genchem/topicreview/bp/ch23/fission.php https://www.aps.org/publications/apsnews/200712/physicshistory.cfm https://www.iaea.org/newscenter/news/pioneering-nuclear-science-discovery-nuclear-fission