Temperature: Difference between revisions
Ayeshaahuja (talk | contribs) |
Ayeshaahuja (talk | contribs) |
||
| Line 64: | Line 64: | ||
<math>{t_f} = {27.576°C} | <math>{t_f} = {27.576°C} | ||
Revision as of 03:15, 27 November 2016
Written by Ju Yup Kim
Edited/Claimed by Ayesha Ahuja
Defining temperature
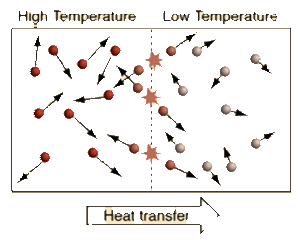
Temperature is measure of average kinetic energy of the particles in a system. Difference between temperature and heat is that heat is the sum of all the kinetic energies of the particles in a system. Adding heat to a system causes its temperature to rise. Newton's zeroth law states that a system reaches a thermal equilibrium when there is no observable change in temperature between a system. Therefore, the change in temperature causes heat to flow from a high temperature system to a low temperature system. When systems with different temperature is in contact, molecules with higher kinetic energy collide with molecules with lower kinetic energy, kinetic energy is passed from the molecules with more kinetic energy to those with less kinetic energy. This molecular level of kinetic energy transfer will happen until average kinetic energy of the particles in each systems reach the average of two.
The relationship between heat transfer and temperature can be modeled with this equation: Q = m c Delta T
Measurement
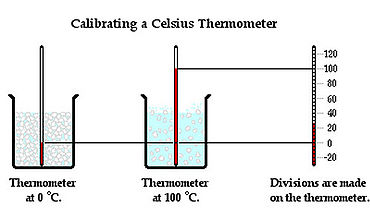
A thermometer is a device used to measure temperature. It is placed in contact with an object and allowed to reach thermal equilibrium with the object. The operation of a thermometer is based on some property, such as volume, that varies with temperature. For example, mercury in a mercury thermometer expands to a degree depending on a temperature of the object and the level of the mercury in side the glass tube rises or descends. Temperature can be measured in numbers by three temperature scales: Celsius, Fahrenheit, and Kelvin. Celsius scale sets freezing point of the water at zero and boiling point at 100, and Fahrenheit scale sets freezing point of the water at 32 degrees and boiling point of the water at 212 degrees. Kelvin scale is designed to go to zero at absolute zero, the minimum temperature.
The relationship between temperature scales: (degrees)K = 273.15 + (degrees)C (degrees)C = (5/9)*((degrees)F-32) (degrees)F = (9/5)*(degrees)C+32
Thermal Energy and Temperature
The temperature and the thermal energy of an object are both measures of how much kinetic energy is acting on the particles of the material. However, thermal energy is the total kinetic energy of every single particle in the material. Temperature is the average speed of the particles in the material. When the temperature of an object is high the particles move fast and are further apart and when the temperature is low they move slower and closer together. When looking at an more than one object the total thermal energy transferred between two objects of different temperatures is referred to as heat ; generally the transfer is from a hotter to a colder temperature.
Heat Transfer
Heat transfers from objects of lower energy to objects of higher energy. There are three main types of heat transfer: conduction, conviction, and radiation. Conduction is seen as the transfer of energy from molecule to molecule. Convection is the energy transferred by the movement and motion of mass. Lastly, radiation is the the transfer of energy by waves.
In physics, conduction is generally the type of heat transfer we focus on. By looking at the formula for thermal energy we can determine the change in energy of a system or the change in temperature of a system.
Examples
(1) On thanksgiving morning your mom cooked a turkey with a mass of 12.55 kg and a temperature of 85°C coming out of the oven. By the time everyone is seated at the table and ready to eat the turkey is now 43°C. Determine the thermal energy of the turkey. The specific heat C for the Turkey is 5.16 J/(gC).
Solution:
[math]\displaystyle{ {∆Ethermal} = {mc∆T} }[/math] where [math]\displaystyle{ {m} }[/math] is the mass of the turkey and [math]\displaystyle{ {c} }[/math] is the specific heat of the turkey and [math]\displaystyle{ {∆T} }[/math] is the change in temperature of the turkey.
[math]\displaystyle{ {∆Ethermal} = {(12.55kg)(5.16J/(gC))(43°C - 84°C) } }[/math]
[math]\displaystyle{ {∆Ethermal} = {-2719.836 J} }[/math]
(2) You take a slice of the turkey (mass: 1.12 kg) at 28°C and place some sauce on top. The sauce has a mass of .044 kg and an initial temperature of 17°C , and a specific heat of 2.2 J/(gC). Looking at the turkey and sauce as a closed system what is the final temperature of the entire system?(T is turkey, S is sauce)
[math]\displaystyle{ {∆Esystem} = {∆EthermalT + ∆EthermalS } }[/math]
[math]\displaystyle{ {∆Esystem} = {m_tc_t(T_f-T_it) + m_sc_s(T_f-T_is) } }[/math]
[math]\displaystyle{ {∆Esystem} = {(m_tc_tT_f-m_tc_tT_it )+ (m_sc_sT_f-m_sc_sT_is) } }[/math]
[math]\displaystyle{ {t_f(m_tc_t+m_sc_s)} = { (m_tc_tT_it) + (m_sc_sT_is) } }[/math]
[math]\displaystyle{ {t_f} = { ((m_tc_tT_it) + (m_sc_sT_is))/(m_tc_t+m_sc_s) } }[/math]
[math]\displaystyle{ {t_f} = { ((1.12kg)(5.16J/gc)(28°C) + ((.044kg)(2.2J/gc)(17°C))/((1.12)(5.16J/gc)+(.044)(2.2J/gc)) } }[/math]
<math>{t_f} = {27.576°C}