Energy Transfer due to a Temperature Difference: Difference between revisions
No edit summary |
No edit summary |
||
| Line 43: | Line 43: | ||
<b>Calculating Heat Transfer</b> | <b>Calculating Heat Transfer</b> | ||
*<math>Q = mC(∆T)</math> | *<math>Q = mC(∆T)</math> | ||
::Q = Heat Added | |||
::m = mass | |||
::C = Specific Heat | |||
::∆T = change in Temperature | |||
===A Computational Model=== | ===A Computational Model=== | ||
Revision as of 18:49, 27 November 2016
This section will be sharing how to calculate energy transfer in systems that are affected by temperature changes. This related to Chapter 7 in the Text Book and Exam 3.
Woong Jun Park
wpark39
Carter Gillon (cgillon3), Fall 2016
The Main Idea
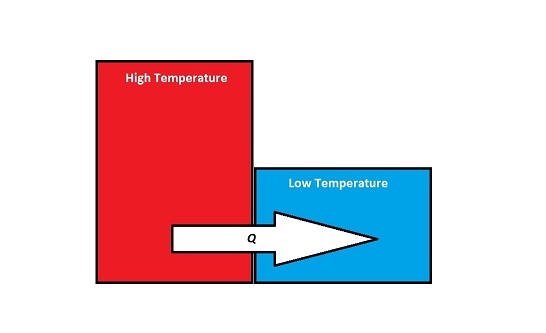
When two objects that have different temperatures come into contact with one another, energy will be transferred from the object with the higher temperature to the object with the lower temperature. Although work is occurring at a microscopic level, since at the point of contact, the individual atoms on the surfaces of the objects are doing work on each other, this energy transfer is not referred to as work, W, because there are no macroscopic forces or displacements. Instead:
- Energy Transfer Due to a Temperature Difference = [math]\displaystyle{ Q }[/math]
Therefore, for an open system, the energy principle is:
- [math]\displaystyle{ ∆E_{system} = Q + W + }[/math] other energy transfers
- Q is used to describe the amount of energy that is exchanged between the system and surroundings when there is a temperature difference between them (microscopic work)
- W is used to describe the energy transfer due to other forces (macroscopic, mechanical work)
- other energy transfers could include matter transfer, mechanical waves, electricity, electromagnetic radiation, etc...
Sign of Q
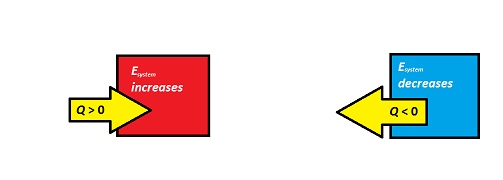
[math]\displaystyle{ Q }[/math] can be positive or negative depending on the energy change.
If energy is transferred from the system to the surroundings (the system is getting colder), then [math]\displaystyle{ Q }[/math] is negative. If energy is transferred from the surroundings to the system (the system is heating up), then [math]\displaystyle{ Q }[/math] is positive. (see the explanation for this in the mathematical model below)
The Steady State When a system is described as being in "steady state", that means that the overall energy of the system, [math]\displaystyle{ E_{system} }[/math], does not change despite the other energy transfers occurring in the system. Also, for a system to be at steady state, the energy flow flowing into the system must be doing so at the same rate as the energy flowing out of the system.
A Mathematical Model
The following are mathematical models that you will use in these calculations:
The Energy Principle
- [math]\displaystyle{ ∆E_{system} = Q + W + }[/math] other energy transfers
Calculating Heat Transfer
- [math]\displaystyle{ Q = mC(∆T) }[/math]
- Q = Heat Added
- m = mass
- C = Specific Heat
- ∆T = change in Temperature
A Computational Model
Check this video out for a brief understanding of how Heat & Temperature relate to Physics.
Heat Transfer
Check this video out for a detailed look into the topic.
Relationship between deltaE and Q+W
Examples
Simple
Middling
Difficult
Example 1:
How much heat energy is required to raise the temperature of 55.0g of water from 25° C to 28.6° C?
Q=mCdeltatT
Q=55g*4.2J/g/K*(28.6° C-25° C)
Q=827.64J
Example 2:
180 grams of boiling water (temperature 100° C, heat capacity 4.2 J/gram/K) are poured into an aluminum pan whose mass is 800 grams and initial temperature 24° C (the heat capacity of aluminum is 0.9 J/gram/K).
After a short time, what is the temperature of the water?
System: Water+Pan
Q = mC(deltaT)
180g*4.2(J/g/K)*(100-T) = 800g*0.9(J/g/K)*(T-22)
T=61.95° C
- In this case, you would assume that the heat capacities for both water and aluminum don't really change with temperature. And also assume the energy transfer between the system and the surroundings was negligible.
Example 3:
Suppose you warm up 510 grams of water (about half a liter, or about a pint) on a stove, and while this is happening, you also stir the water with a beater, doing 6104 J of work on the water. After the large-scale motion of the water has dissipated away, the temperature of the water is observed to have risen from 23°C to 82°C.
A) What was the change in the thermal energy of the water?
deltatE = mCdeltaT
510g*4.2(J/g/K)(82-23)
deltatE = 126378 J
B) Taking the water as the system, how much energy transfer due to a temperature difference (microscopic work) Q was there across the system boundary?
Q = E-W
126378J - 6e4J
Q = 66378J
C) Taking the water as the system, what was the energy change of the surroundings?
deltaE_system = - deltaE_surroundings
deltaE_surroundings = -126378
Connectedness
I am a Business, Pre-Dental student so health topics have always been very interesting to me. Although this topic may not relate directly to my major, it does have real world applications in the health field.
Specific heat and this transfer of energy can be applied to taking patient's temperatures using a thermometer. There is liquid and material that is inside the thermometer that a have low specific capacities. This allows for a very precise measurement of the temperature. This is not necessarily
Another industrial application can e seen in engine parts that expand and contract because of the constant change in heat within the engine.
History
The history of heat and work can be dated back to 1789 when the French scientist Antoine Lavoisier created a new theory - the phlogiston theory on Chemistry that basically negated all previous findings of combustion. He gave heat a meaning and definition that led to the interpretation of heat that is accepted today.
The modern interpretation is what we hold true in the example questions that we used earlier Q = mCdeltatT.
In 1798, Benjamin Thompson - minister for war and police in the German state of Bavaria - wanted to figure out where all the heat from the cannons were coming from. He observed that the surroundings of the cannon got hotter and not colder. He hypothesized that some of the mechanical work done on the cannon was converted to heat.
In 1849, English physicist James Prescott Joule published his work and findings on the conversion of work to heat that Thompson started. He formulated work equivalent of heat. 1 newton meter of work = 0.241 calories of heat.
In 1850, German physicist Clausius published his works on how conserved quantity is neither heat nor work, but a combination of both. He named this Energy and that is where we get the macroscopic equation deltaE = Q - W.
See also
Relevant material and additional reading can be looked into the history of Joule and the laws of thermodynamics.
Further reading
Specific Heat: Britannica
Dependence of Heat Capacity on Thermal History: Nature Publishing Group
Environmental Research Web | Uneven Climate Change Due to Atmospheric Heat Capacity
External links
USGS Water Science School
Science Direct | Determination of specific heat capacity by transient plane source
Heat Transfer
Relationship between deltaE and Q+W
References
"A Brief History of Heat and Work." A Brief History of Heat and Work. N.p., n.d. Web. 10 Nov. 2015.\
"Application of Specific Heat Capacity" : Application of Specific Heat Capacity. N.p., n.d. Web. 10 Nov. 2015.
"CHAPTER 4: HEAT." : 4.2 Specific Heat Capacity. N.p., n.d. Web. 10 Nov. 2015.
Division of Building Technology, Chalmers University of Technology, Gothenburg SE-412 96, Sweden Received 23 August 2013, Revised 3 September 2013, Accepted 10 September 2013, Available online 22 October 2013
"Re: What Are Some Practical Uses of Determining the Specific Heat of a Metal?" Re: What Are Some Practical Uses of Determining the Specific Heat of a Metal? N.p., n.d. Web. 10 Nov. 2015.