Kinds of Matter: Difference between revisions
No edit summary |
No edit summary |
||
| Line 19: | Line 19: | ||
=== | ===Liquid=== | ||
Gases bounce everywhere and spread out but many liquids want to stick together due to intermolecular forces called cohesive (sticky) forces that act to pull the molecules together. These attractive forces exist | |||
===Atoms and Nuclei=== | ===Atoms and Nuclei=== | ||
Revision as of 23:21, 28 November 2016
Claimed by Kristen Sparks Claimed by Pranusha Atuluru (Fall 16)
This topic covers the Different Kinds of Matter.
The Main Idea
No matter how how big or small the matter, physics can be applied to all objects. States of matter are differentiated or determined by significant changes that occur due to the alteration of an important property such as specific heat capacity, pressure, or temperature. Specific heat capacity is the heat required to raise the temperature of a unit mass by a given amount (usually by one degree). Pressure is a continuous physical force exerted on or against an object and temperature is the degree of heat present in a substance or object. In different states of matter, things only move from one phase to another through physical means and not chemical where the same chemical properties of the substance is the same.
Solids
Solids is a sample of matter that retains its shape and size even when it is not confined by another object and that occupies a specific area and volume. They are difficult to deform because they are resistant to changes of shape or volume. The molecules in a solid object are usually as confined and packed together as possible or as much as the repulsive forces between the molecules allow. When a solid is heated or when energy is put into a solid, the molecules gain kinetic energy and eventually overcome the forces that hold them in place. If enough kinetic energy is gained, it leads to a phase change where the solid becoming a gas or a liquid.

The process where a solid becomes a liquid is called melting. The special temperature at which a solid becomes a liquid is melting point. For example, the melting point of ice is 0 where the ice becomes liquid water. There is also a process where solid becomes a gas called sublimation. This occurs with dry ice (solid CO2) that becomes gas as soon as it is put out in room temperature.
There are two main categories of solids called crystalline solids and amorphous solids. Crystalline solids are solids where the atoms of molecules that make up the solid are arranged in a neat, well-defined arrangement. Four types of crystalline solids include ionic, molecular, atomic, and metallic solids. Amorphous solids are those in which the molecules do not have much arrangement. Although the molecules are close together and have little freedom to move, they are not as well-defined in arrangement as crystalline solids.
Liquid
Gases bounce everywhere and spread out but many liquids want to stick together due to intermolecular forces called cohesive (sticky) forces that act to pull the molecules together. These attractive forces exist
Atoms and Nuclei
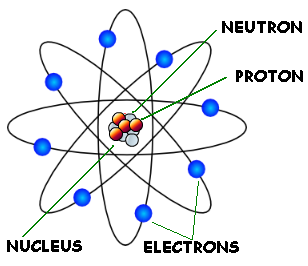
All matter is made of atoms. To understand the properties of matter around us we look at atomic properties and interactions. Atomic interactions can be attributed to the attractive and repulsive forces due to the different parts of an atom which are: protons, electrons, and neutrons. Protons are positively charged particles, electrons are negative, and neutrons have no charge. Protons and neutrons make up a small, dense center called a nucleus. Around the nucleus are electrons, whose negative forces are attracted to the positive center.
The number of neutrons and protons in specific chemical elements can be found on the periodic table.
Molecules and Solids
When atoms bond together molecules are formed. Molecules can be made up of any number of chemical elements, but as a whole the molecule's properties differ from the properties of its subelements.
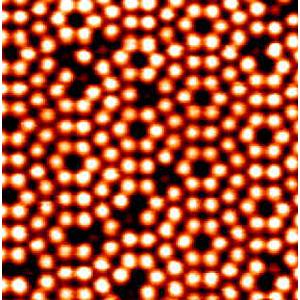
Solids are rigid objects made up of molecules. Scanning tunneling microscopes (STMs) are used to see molecules and atoms that make up everyday solids. STMs can observe patterns and irregularities that may occur on the surface of solids, which have helped scientists understand more about what happens on the atomic level of matter.
Liquids and Gases
Liquids are formed when solids are heated. Atoms begin to vibrate too fast to maintain their normal rigid positions. If the temperature increases enough atoms will start to shift past one another therefore becoming a liquid. If the temperature is further increased the sliding of atoms will turn into interatomic bond breaking which will lead to gas formation.
Planets, Stars, Solar Systems and Galaxies
Plants and stars make up solar systems, and multiple solar systems make up galaxies.
Point Particles
Point particles are often used to analyze more complicated/large objects. Physicists assume that these objects have been compressed into structures where size, shape and internal structure are not taken into consideration: a tiny speck with equivalent mass as the object its representing.
Examples
Atom
Crystalline Solid
STM Image
Connectedness
The different kinds of matter are important to understanding how physics can be applied to everyday life. It helps connect the misunderstood atomic and subatomic level to something that everyone can understand. More advanced physics will correlate atomic bonds to springs, a juxtaposition that helps explain a complex interaction with a simple explanation.
History
The first theories on atomic and subatomic particles began with J. J. Thompson in 1897, when he discovered electrons. An idea made the scientific community realize that atoms were not the smallest particles of matter. Then in 1909 Ernest Rutherford started experimenting with gold and alpha particles, and experiment that lead to the discovery of nuclei.
These fundamental discoveries lead to the deep understanding of the atomic world that scientists have today. Without them the properties of matter and physics would be a grayer area of science.
See also
External links
http://www.livescience.com/46506-states-of-matter.html
References
Matter and Interactions By Ruth W. Chabay, Bruce A. Sherwood - Chapter 1
http://www.sparknotes.com/testprep/books/sat2/physics/chapter19section2.rhtml