The Energy Principle: Difference between revisions
Parkercoye (talk | contribs) |
Parkercoye (talk | contribs) (→Simple) |
||
| Line 127: | Line 127: | ||
'''Two cars are in a parking lot. The first car crashes into the second car, which is initially at rest. The final kinetic energy of the first car is 50J and the final kinetic energy of the second car is 30J. What is the initial kinetic energy of the system?''' | '''Two cars are in a parking lot. The first car crashes into the second car, which is initially at rest. The final kinetic energy of the first car is 50J and the final kinetic energy of the second car is 30J. What is the initial kinetic energy of the system?''' <br> | ||
'''Step 1: Draw the problem and write out what you know''' | '''Step 1: Draw the problem and write out what you know'''<br> | ||
[[File:Collisionproblem1.png]] | [[File:Collisionproblem1.png]] | ||
'''Step 2: Apply the Energy Principal''' | '''Step 2: Apply the Energy Principal''' <br> | ||
[[File:Collisionproblem2.jpg]] | |||
Remember - Kinetic energy is a scalar, not a vector - express your answer as such! | |||
===Difficult=== | ===Difficult=== | ||
Revision as of 18:49, 22 May 2019
The Energy Principle
The basis of the energy principle can be described with the statement, "energy can neither be created nor destroyed." Thus, energy may only flow from one system to its surroundings. The observable universe is comprised of this system and everything else not in the system called the surroundings. The energy principle is used to describe changes in energy on a system. These energies can take numerous different forms including Kinetic Energy, Potential Energy, Chemical Energy, Rest Energy, and Thermal Energy. Creating boundaries allows for the conservation of these different types energy in that energy is not lost nor is it gained, it is simply transferred into other forms. Any energy moving over the boundaries therefore can be accounted for having been transferred from a system to its surroundings and vice versa. This notion is the core idea informing the Energy Principle: The change in energy of the system should be equal to the energy inputs from surroundings.
The Main Idea
The Energy Principle, also referred to as The First Law of Thermodynamics, defines the transfer of energy between systems. It is defined with the fact that the change of the energy of system is equal to the work surrounding in addition to heat transfers from the surroundings as well. This principle can be modeled by the equations:
(1) E = Q+W
(2) E(system) + E (surroundings) = 0
You can see that these equations (particularly equation 2) describe Conservation of Energy, which is a main idea in physics, particularly in this course!
How will we use the energy principal?
In this course, we can apply the Energy Principal to many different scenarios. We can track how energy takes on different forms during an event. Whether its potential energy being turned into kinetic energy when a woman goes bungee jumping, or electrical energy turning into heat as you use your laptop, the transformation of energy can tell us a lot about a given scenario.
A basic outline for how to solve a problem using the energy principal:
(1) Determine the different types of energy associated with the problem
(2) Determine if there are values for Q and W (heat and work) associated with the problem
(3) Determine the equations for each type of energy identified
(4) Plug these into the energy equation and solve for the unknowns
Don’t worry if you’re not sure how these steps are worked out yet – you will soon!
Single Particle vs Multi Particle Systems
As with many concepts in physics, calculating the energy for the multi particle system is exactly the same as calculating the energy for a single particle system – except for the fact that you will need to account for multiple particles.
For example, If a system of one particle has a kinetic energy of 100J, then the total kinetic energy for that system is 100J. If another system consists of three particles, each with a kinetic energy of 20J, then the total kinetic energy of this system is 60J.
The same idea applies for gravitational potential energy, electric potential energy, etc.
Here is that same concept in another form:
KEfinal + U(potential energy)final = Work(surroundings) + Q(heat) + KEintial + U(potential energy)initial
For a multi-particle system: Esystem=(K1+K2+K3+…)+(U1,2+U1,3+U2,3+…)
Click here for a demonstration of the Energy Principle
Mathematical Models
These are the main equations you will be using to solve problems using the Energy Principal. We will add more equations later when describing the differing types of energy (kinetic, potential gravitational, energy of a single particle approaching the speed of light, etc) but for now, just focus on these.
The Energy Principle
EQ 1: [math]\displaystyle{ {∆E} = {Q + W} }[/math] where [math]\displaystyle{ {Q} }[/math] is heat and [math]\displaystyle{ {W} }[/math] is the amount of work acting on the system.
EQ 2: [math]\displaystyle{ {∆E} = {∆K + ∆E_{Rest} + ∆U + ∆E_{Thermal}} }[/math] - the different types of energy that can be associated with a given particle in a system. Not all have to be present. These terms will vary based on the internal properties of the system being observed.
A Computational Model
These gifs demonstrate the energy principal from a Conservation of Energy standpoint. As the ball on a spring approaches the equilibrium point, the kinetic energy increases and the spring potential decreases. These values will oscillate, but the total energy will stay constant! This demonstration was written in GlowScript and iteratively updates the ball's momentum while taking into account the spring force.
Energy of a single particle
A lot of problems in introductory physics classes involve objects in the macroscopic world: calculating trajectories of cannonballs, maximum velocities of race cars, or angles of deflection of two hockey pucks. There is, however, more applications for the energy principal in the microscopic world. Sometimes when dealing with particles very very small (around atomic scale size), our calculations will be a bit different from what we're used to, but they're actually fairly similar when broken down. Lets begin by defining some terms and constants which will be useful later on:
Types of point particles:
- 1. Electrons
- 2. Protons
- 3. Neutrons
Masses of point particles:
- 1. Electron mass = 9.109 e -31 kg
- 2. Proton mass = 1.6726 e -27 kg
- 3. Neutron mass = 1.6750 e -27 kg
Here it is: probably Einstein's most famous equation: calculating the rest energy of a particle using its mass and the speed of light (c = 3e8 m/s).
[math]\displaystyle{ E_{rest}=mc^2 }[/math]
- Note that rest energy is not dependent on particle speed
The energy of a particle with mass m:
[math]\displaystyle{ E_{particle}=γmc^2 }[/math]
- Note here that the kinetic energy of a point particle is just like the kinetic energy of, say, a basketball except for a constant called gamma (also called the relativistic correction factor).
The kinetic energy K of a particle:
[math]\displaystyle{ K=γmc^2-mc^2 }[/math]
- Note that kinetic energy in this form has to be used when the particle is moving near the speed of light
- If v<<c, then [math]\displaystyle{ K=\frac{1}{2}mv² }[/math]
Change in rest energy can be useful when finding the energy change of a decay and of fission
- Uranium nucleus that undergoes fission splits into a bunch of neutrons and a few daughter nuclides.If you trap them all and weigh them the total mass is slightly less than the Uranium nucleus you started with. The missing mass has been converted to energy
Example:
A proton moves at 0.950c. Calculate its (a) rest energy, (b) total energy, and (c) kinetic energy.
- (a) [math]\displaystyle{ E_{rest}=mc^2 }[/math] = (1.67e-27 kg)(3e8 m/s)^2 = 1.5e-10 J
- (b) [math]\displaystyle{ E_{particle}=γmc^2 }[/math] = (1.5e-10 J)/((1-(0.950c/c)^2)^(1/2)) = 4.81e-10 J
- (c) [math]\displaystyle{ K=γmc^2-mc^2 }[/math] = 4.81e-10 J - 1.50e-10 J = 3.31e-10 J
Examples
Simple
Car Crash:
Two cars are in a parking lot. The first car crashes into the second car, which is initially at rest. The final kinetic energy of the first car is 50J and the final kinetic energy of the second car is 30J. What is the initial kinetic energy of the system?
Step 1: Draw the problem and write out what you know

Step 2: Apply the Energy Principal
Remember - Kinetic energy is a scalar, not a vector - express your answer as such!
Difficult
A 90 kg rock is moving with a speed of 4.7 m/s. A machine tries to slow it down by pushing it in the opposite direction of its movement for 2.7 m at an average force of 215 N. What is the speed of the rock after the machine stops pushing the rock?
Solution:
There is no transfer of heat so Q is eliminated from The Energy Principle equation. The equation you would use is ΔEsys= W. Additionally, the work is negative in this scenario because the force is in the opposite direction of momentum. There is no potential energy of any sort. Work = Force(exerted by surroundings/machine) * distance.
Thus,
ΔE = Work
ΔU = 0
ΔE = W = ΔKE + ΔU
Work = KEf - KEi
Work = Force * distance = (215 N) * (2.7 m) = 580.5 N*m
Velocityi = 0, Velocityf = ?
1/2*m*(Velocityf)^2 = -580.5 + 1/2*m*(Velocityi)^2
Masses cancel out again
1/2*(90 kg)*(Velocityf)^2 = -580.5 + 1/2*(90kg)(4.7 m/s)^2
Therefore after solving for Velocityf,
Velocityf = 3.0315 m/s
Connectedness
The Energy Principle can be used for a variety of situations; the fact that it can tell us something about the work acting on a system with only knowing about what energies are present (and vice versa) is what makes this such a fundamental principle. The energy principle is also used to describe the conservation of energy because energy doesn't just disappear, it's converted into a different form. The initial and final total energies of a system should be the same because energy is never lost, just converted into a different form. These sorts of energy balances are used not only in physics, but are integrated into many areas of engineering where the manufacturing of products is supplied by different types of energy such as thermal, electric, gravitational, etc.
Example: Energy Balances in Chemical Engineering
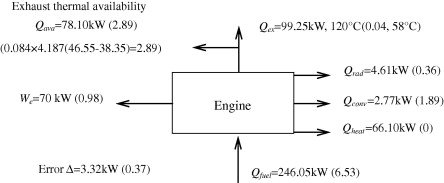
History
The concept of energy and its connection to the amount of work performed goes all the way back to the age of steam engines; physicists and engineers came up with this notion to determine the mechanical and thermal efficiency of their machines. In the 1850's, people like William Thomson and William Rankine began to come up with terms like 'kinetic energy' and 'potential energy' to model the different types of observed forces. After the 1920's, this study of science became to be known as thermodynamics, the science of energy transformations. This led to the laws of thermodynamics, one of which relates to the conservation of energy. William Rankine was the first to discuss the law of the conservation of energy in relation to a more general "energy principle". His discussions and work in this field defined the relationships between energy that we now consider the Energy Principle.
See also
Potential Energy
Rest Mass Energy
Kinetic Energy
Work
Thermal Energy
Gravitational Potential Energy
Conservation of Energy
Spring Potential Energy
Further reading
Matter and Interactions By Ruth W. Chabay, Bruce A. Sherwood - Chapter 6
External links
http://hyperphysics.phy-astr.gsu.edu/hbase/conser.html#coneng
http://hyperphysics.phy-astr.gsu.edu/hbase/enecon.html
https://www.youtube.com/watch?v=-tNQKn0EfBo
https://www.youtube.com/watch?v=30o4omX5qfo
https://www.youtube.com/watch?v=LNk2mUbnKus
https://www.youtube.com/watch?v=5Vfl1uX6kxM
References
http://p3server.pa.msu.edu/coursewiki/doku.php?id=183_notes:define_energy
http://www.texample.net/tikz/examples/earth-orbit/
https://en.wikipedia.org/wiki/Conservation_of_energy
https://en.wikipedia.org/wiki/History_of_energy
Chabay, Ruth W., and Bruce A. Sherwood. Matter and interactions. Hoboken: Wiley, 2015. Print.




