Franck-Hertz Experiment: Difference between revisions
Tbennett37 (talk | contribs) No edit summary |
Tbennett37 (talk | contribs) No edit summary |
||
| Line 3: | Line 3: | ||
==Main Idea== | ==Main Idea== | ||
James Franck and Gustav Hertz were German physicists that helped to prove the [[Quantum Theory]] using their collisional excitation experiment in 1914. The quantum theory states that atoms have discrete levels made up of electrons of specific kinetic energies for each level. To confirm this, the Franck-Hertz duo created an accelerating apparatus, which simply sends electrons with varying kinetic energies through a vapor (in this case, mercury vapor) and then collects the electrons that weren’t picked up by the atoms in the vapor. The results showed that once the electrons reached the threshold kinetic energy of 4.9 eV, most of them were picked up by the atoms, and they didn’t reach the collector. This proved that the first level of the mercury atoms needs electrons with at least 4.9 eV to be absorbed. This technique was used with other elements to find their own threshold kinetic energies. | [[File:app.gif|200px|thumb|right|alt text]] [[File:update.gif|200px|thumb|right|alt text]] James Franck and Gustav Hertz were German physicists that helped to prove the [[Quantum Theory]] using their collisional excitation experiment in 1914. The quantum theory states that atoms have discrete levels made up of electrons of specific kinetic energies for each level. To confirm this, the Franck-Hertz duo created an accelerating apparatus, which simply sends electrons with varying kinetic energies through a vapor (in this case, mercury vapor) and then collects the electrons that weren’t picked up by the atoms in the vapor. The results showed that once the electrons reached the threshold kinetic energy of 4.9 eV, most of them were picked up by the atoms, and they didn’t reach the collector. This proved that the first level of the mercury atoms needs electrons with at least 4.9 eV to be absorbed. This technique was used with other elements to find their own threshold kinetic energies. | ||
===A Mathematical Model=== | ===A Mathematical Model=== | ||
Revision as of 15:37, 29 November 2015
Claimed and created by Tyler Bennett
Main Idea
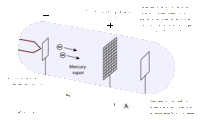
James Franck and Gustav Hertz were German physicists that helped to prove the Quantum Theory using their collisional excitation experiment in 1914. The quantum theory states that atoms have discrete levels made up of electrons of specific kinetic energies for each level. To confirm this, the Franck-Hertz duo created an accelerating apparatus, which simply sends electrons with varying kinetic energies through a vapor (in this case, mercury vapor) and then collects the electrons that weren’t picked up by the atoms in the vapor. The results showed that once the electrons reached the threshold kinetic energy of 4.9 eV, most of them were picked up by the atoms, and they didn’t reach the collector. This proved that the first level of the mercury atoms needs electrons with at least 4.9 eV to be absorbed. This technique was used with other elements to find their own threshold kinetic energies.
A Mathematical Model
What are the mathematical equations that allow us to model this topic. For example [math]\displaystyle{ {\frac{d\vec{p}}{dt}}_{system} = \vec{F}_{net} }[/math] where p is the momentum of the system and F is the net force from the surroundings.
A Computational Model
How do we visualize or predict using this topic. Consider embedding some vpython code here Teach hands-on with GlowScript
Examples
Be sure to show all steps in your solution and include diagrams whenever possible
Simple
Middling
Difficult
Connectedness
- How is this topic connected to something that you are interested in?
- How is it connected to your major?
- Is there an interesting industrial application?
History
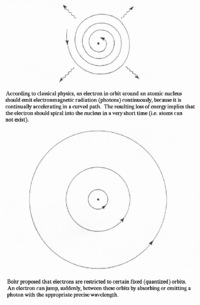
In 1911, Ernest Rutherford created a model of the atom which was proven wrong in the next couple of years. His model displayed only a single level that spiraled about the nucleus of the atom. Niels Bohr noted that this model would mean that atoms could not exist, as the electrons would spiral into the nucleus. Bohr then came up with his own model in 1913 that consisted of different shells outside of the atom. The electrons could jump shells and they would have constant quantized orbits. The Franck-Hertz experiment confirmed this model in showing that atoms can only obtain electrons when they contain enough kinetic energy to be able to jump onto the atoms’ shells.
See also
Quantum Theory Ernest Rutherford Niels Bohr
Further reading
Books, Articles or other print media on this topic
External links
Internet resources on this topic
References
This section contains the the references you used while writing this page