Scattering: Collisions in 2D and 3D: Difference between revisions
No edit summary |
|||
| Line 50: | Line 50: | ||
==Examples== | ==Examples== | ||
===Simple=== | ===Simple=== | ||
'''Question''' | |||
'''Answer''' | |||
===Scattering in 2D=== | ===Scattering in 2D=== | ||
| Line 58: | Line 62: | ||
As alpha particles approach the Rutherford atom in the center, they are repelled from it. The closer the alpha particles are to the atom in the center, the more they are repelled. These closer alpha particles are repelled to sometimes angles of greater than 100°. | As alpha particles approach the Rutherford atom in the center, they are repelled from it. The closer the alpha particles are to the atom in the center, the more they are repelled. These closer alpha particles are repelled to sometimes angles of greater than 100°. | ||
==Connectedness== | ==Connectedness== | ||
Revision as of 21:16, 1 July 2019
Edwin Zhao - Spring 17
The Main Idea
Scattering (Rutherford Scattering) is a type of experiment that is used to study the structure and behavior of atoms, nuclei, and other small particles. This allows us to understand small particles on a greater level.
Unlike normal collisions, atomic and nuclear collisions are far too small to observe the curving trajectories of the interacting particles. The only things that can be noticed are the initial and final states of the interaction. Therefore, the method of observing scattering consisted of alpha particles from a radioactive source striking a thin gold foil. Since it is only a very small number of atoms are observed to scatter after contact with the gold, it can be determined that the majority of the material is positively charged, and when the alpha particle (positive) travels through and approaches close enough to the nucleus, it will be repelled and then "scatter" by a large angle.
By finding the back-scattering, it shows that atoms are arranged tightly together. Scattering experiments are useful in the world of collisions to be able to study the minute details in the structure of atoms, nuclei, and other tiny particles as they interact with one another.
After collision with the gold nucleus, the alpha particle gets deflected some angle θ. The gold nucleus recoils at some angle Φ. It is important to pick optimal time frames ("before" early enough and "after" late enough) so that the two particles are far enough away from each other to minimize their electric potential energies. The speeds in this approach are small in comparison to the speed of light.
The case of Rutherford scattering of alpha particles with gold nuclei is an example of elastic scattering because the initial and final velocities and energies stay are the same.
Impact Parameters
An impact parameter is the distance between centers of the colliding objects perpendicular to the incoming velocity. Impact parameter is often denoted by the variable b.
A head-on collision has an impact parameter of zero, and with equal masses fully transfers the momentum- such as with Newton's Cradle. As the impact parameter gets smaller the collision has a larger effect, and an even large deflection angle (scattering).
A Mathematical Model
[math]\displaystyle{ p_{xi} = p_{xf} }[/math]
[math]\displaystyle{ p_1 = p_3 cosθ + p_4 cosΦ }[/math]
[math]\displaystyle{ p_{yi} = p_{yf} }[/math]
[math]\displaystyle{ 0 = p_3 cos(90° - θ) + p_4 cos(90° + Φ) }[/math]
[math]\displaystyle{ K_f = K_i }[/math]
[math]\displaystyle{ \frac{p^2_1}{2m} = \frac{p^2_3}{2m} + \frac{p^2_4}{2M} }[/math]
Where [math]\displaystyle{ p_1, p_3, }[/math] and [math]\displaystyle{ p_4 }[/math] are all magnitudes of the momenta. It can be remembered that the gold nucleus is initially at rest.
The directions of cosine are used to express vector components to the x-axis.
ex. Final momentum of gold nucleus [math]\displaystyle{ {= \vec{p_4} = |\vec{p_4}| \lt cosΦ, cos(90° + Φ), 0\gt } }[/math]
A Computational Model
Examples
Simple
Question
Answer
Scattering in 2D
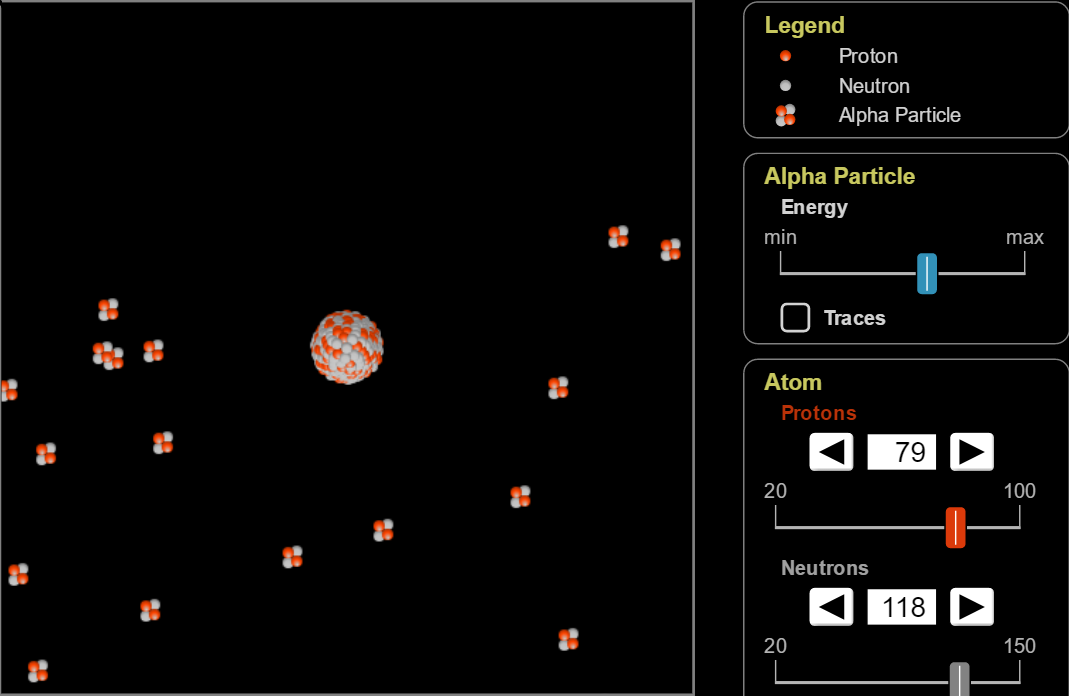 [Rutherford Scattering Example]
[Rutherford Scattering Example]
As alpha particles approach the Rutherford atom in the center, they are repelled from it. The closer the alpha particles are to the atom in the center, the more they are repelled. These closer alpha particles are repelled to sometimes angles of greater than 100°.
Connectedness
History
In 1871 Lord Rayleigh published a paper on scattering. Rayleigh scattering is the dispersion of electromagnetic radiation by particles that have a minute radius less than approximately 1/10 the wavelength. It laid the foundation to research on scattering and information we have today.
In Ernest Rutherford's laboratory with Hans Geiger and Ernest Marsden, they experimented with tests of scattering alpha particles with thin metal foils. In 1909, Geiger and Marsden discovered scattering through their gold foil experiments. From this, they discovered that some alpha particles scattered at angles greater than 90°.
See also
Further reading
Matter and Interactions, Volume I: Modern Mechanics, 4th Edition. (Chapter 10.6)
External links
References
Chabay, Ruth W., Bruce Sherwood. Matter and Interactions, Volume I: Modern Mechanics, 4th Edition. Wiley, 19/2014.
