Temperature: Difference between revisions
| Line 1: | Line 1: | ||
==Main Idea== | ==Main Idea== | ||
[[File:Tempfigure.gif|300px|thumb|right(position)|]] | |||
Temperature is the measure of the Average Kinetic Energy of the particles in a system. The difference between Temperature, Thermal Energy, and Heat is: | |||
<blockquote>''Thermal Energy is the sum of all the kinetic energies of the particles in a system''</blockquote> | |||
<blockquote>''Heat is the transfer of Thermal Energy''</blockquote> | |||
Applying Heat to a system usually causes its Temperature to rise; the loss of Heat by a system typically corresponds to decrease in Temperature. The Zeroth Law of Thermodynamics states: | |||
<blockquote>''If system A is in Thermodynamic Equilibrium with system B, and if system B is in Thermodynamic Equilibrium with system C, then system A is also in Thermodynamic Equilibrium with system C''</blockquote> | |||
Thermodynamic Equilibrium is defined as: | |||
<blockquote>''When a system has no Heat exchange with itself''</blockquote> | |||
Thus, a system's Temperature is the same throughout when it is in Thermodynamic Equilibrium. | |||
We see from the definition of Thermodynamic Equilibrium and The Zeroth Law of Thermodynamics that a Temperature difference is needed for Heat to flow from a warmer Temperature system to a cooler Temperature system. When systems with different Temperatures are in contact, the molecules in the system with higher average kinetic energy collide with the molecules of the system with lower average kinetic energy; kinetic energy is passed from the molecules with more kinetic energy to those with less kinetic energy. This molecular level of kinetic energy transfer will happen until the average kinetic energy of the particles in each system reaches the weighted average of the two systems. This idea can be applied to a multi-system interaction as well. | |||
===Mathematical Model=== | ===Mathematical Model=== | ||
[[File:Thermometer11.jpg|370px|thumb|right(position)|]] | |||
A thermometer is a device used to measure the Temperature of a system. It is placed in contact with an object and allowed to reach Thermal Equilibrium with the object, and thus the same Temperature. The operation of a thermometer is based on some property, such as volume, that varies with Temperature. For example, mercury in a mercury thermometer expands to a degree depending on a temperature of the object and the level of the mercury inside the glass tube rises or descends due to this. | |||
Temperature can be measured in numbers by three Temperature scales: Celsius, Fahrenheit, and Kelvin. The Celsius scale sets the freezing point of the water at 0 degrees and the boiling point of water at 100 degrees. The Fahrenheit scale sets the freezing point of water at 32 degrees and the boiling point of the water at 212 degrees. The Kelvin scale is designed to go to 0 at ''absolute zero'', the minimum possible temperature in the Universe. | |||
The three Temperature scales are related by the following: | |||
:::<math>°K = 273.15 + °C</math> | |||
:::<math>°C = \left(\frac{5}{9}\right)(°F - 32) \quad \And \quad °C = °K - 273.15</math> | |||
:::<math>°F = \left(\frac{9}{5}\right)(°C + 32)</math> | |||
The relationship between Heat transfer and Temperature can be modeled with this equation: | |||
:::<math>Q = mCT</math> | |||
===Computational Model=== | ===Computational Model=== | ||
:'''Insert Computational Model here''' | |||
==Examples== | ==Examples== | ||
Revision as of 21:14, 5 August 2019
Main Idea
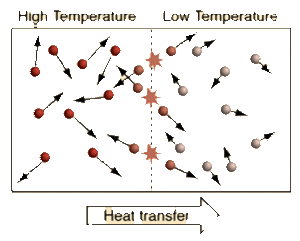
Temperature is the measure of the Average Kinetic Energy of the particles in a system. The difference between Temperature, Thermal Energy, and Heat is:
Thermal Energy is the sum of all the kinetic energies of the particles in a system
Heat is the transfer of Thermal Energy
Applying Heat to a system usually causes its Temperature to rise; the loss of Heat by a system typically corresponds to decrease in Temperature. The Zeroth Law of Thermodynamics states:
If system A is in Thermodynamic Equilibrium with system B, and if system B is in Thermodynamic Equilibrium with system C, then system A is also in Thermodynamic Equilibrium with system C
Thermodynamic Equilibrium is defined as:
When a system has no Heat exchange with itself
Thus, a system's Temperature is the same throughout when it is in Thermodynamic Equilibrium.
We see from the definition of Thermodynamic Equilibrium and The Zeroth Law of Thermodynamics that a Temperature difference is needed for Heat to flow from a warmer Temperature system to a cooler Temperature system. When systems with different Temperatures are in contact, the molecules in the system with higher average kinetic energy collide with the molecules of the system with lower average kinetic energy; kinetic energy is passed from the molecules with more kinetic energy to those with less kinetic energy. This molecular level of kinetic energy transfer will happen until the average kinetic energy of the particles in each system reaches the weighted average of the two systems. This idea can be applied to a multi-system interaction as well.
Mathematical Model
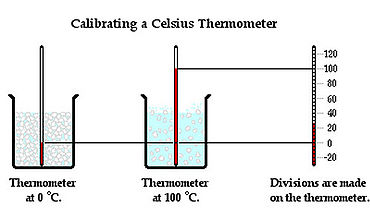
A thermometer is a device used to measure the Temperature of a system. It is placed in contact with an object and allowed to reach Thermal Equilibrium with the object, and thus the same Temperature. The operation of a thermometer is based on some property, such as volume, that varies with Temperature. For example, mercury in a mercury thermometer expands to a degree depending on a temperature of the object and the level of the mercury inside the glass tube rises or descends due to this.
Temperature can be measured in numbers by three Temperature scales: Celsius, Fahrenheit, and Kelvin. The Celsius scale sets the freezing point of the water at 0 degrees and the boiling point of water at 100 degrees. The Fahrenheit scale sets the freezing point of water at 32 degrees and the boiling point of the water at 212 degrees. The Kelvin scale is designed to go to 0 at absolute zero, the minimum possible temperature in the Universe.
The three Temperature scales are related by the following:
- [math]\displaystyle{ °K = 273.15 + °C }[/math]
- [math]\displaystyle{ °C = \left(\frac{5}{9}\right)(°F - 32) \quad \And \quad °C = °K - 273.15 }[/math]
- [math]\displaystyle{ °F = \left(\frac{9}{5}\right)(°C + 32) }[/math]
The relationship between Heat transfer and Temperature can be modeled with this equation:
- [math]\displaystyle{ Q = mCT }[/math]
Computational Model
- Insert Computational Model here
Examples
Simple
Middling
Difficult
Connectedness
History
See also
Further reading
External links
References
Thermal Energy and Temperature
The temperature and the thermal energy of an object are both measures of how much kinetic energy is acting on the particles of the material. However, thermal energy is the total kinetic energy of every single particle in the material. Temperature is the average speed of the particles in the material. When the temperature of an object is high the particles move fast and are further apart and when the temperature is low they move slower and closer together. When looking at an more than one object the total thermal energy transferred between two objects of different temperatures is referred to as heat ; generally the transfer is from a hotter to a colder temperature.
Heat Transfer
Heat transfers from objects of lower energy to objects of higher energy. There are three main types of heat transfer: conduction, convection, and radiation. Conduction is seen as the transfer of energy from molecule to molecule. Convection is the energy transferred by the movement and motion of mass. Lastly, radiation is the the transfer of energy by waves.

In physics, conduction is generally the type of heat transfer we focus on. By looking at the formula for thermal energy we can determine the change in energy of a system or the change in temperature of a system.
Examples
Simple
(1) On thanksgiving morning your mom cooked a turkey with a mass of 12.55 kg and a temperature of 85°C coming out of the oven. By the time everyone is seated at the table and ready to eat the turkey is now 43°C. Determine the thermal energy of the turkey. The specific heat C for the Turkey is 5.16 J/(gC).
Solution:
[math]\displaystyle{ {∆Ethermal} = {mc∆T} }[/math] where [math]\displaystyle{ {m} }[/math] is the mass of the turkey and [math]\displaystyle{ {c} }[/math] is the specific heat of the turkey and [math]\displaystyle{ {∆T} }[/math] is the change in temperature of the turkey.
[math]\displaystyle{ {∆Ethermal} = {(12.55kg)(5.16J/(gC))(43°C - 84°C) } }[/math]
[math]\displaystyle{ {∆Ethermal} = {-2719.836 J} }[/math]
Difficult
(2) You take a slice of the turkey (mass: 1.12 kg) at 28°C and place some sauce on top. The sauce has a mass of .044 kg and an initial temperature of 17°C , and a specific heat of 2.2 J/(gC). Looking at the turkey and sauce as a closed system what is the final temperature of the entire system?(T is turkey, S is sauce)
[math]\displaystyle{ {∆Esystem} = {∆EthermalT + ∆EthermalS } }[/math]
[math]\displaystyle{ {∆Esystem} = {m_tc_t(T_f-T_it) + m_sc_s(T_f-T_is) } }[/math]
[math]\displaystyle{ {∆Esystem} = {(m_tc_tT_f-m_tc_tT_it )+ (m_sc_sT_f-m_sc_sT_is) } }[/math]
[math]\displaystyle{ {t_f(m_tc_t+m_sc_s)} = { (m_tc_tT_it) + (m_sc_sT_is) } }[/math]
[math]\displaystyle{ {t_f} = { ((m_tc_tT_it) + (m_sc_sT_is))/(m_tc_t+m_sc_s) } }[/math]
[math]\displaystyle{ {t_f} = { ((1.12kg)(5.16J/gc)(28°C) + ((.044kg)(2.2J/gc)(17°C))/((1.12)(5.16J/gc)+(.044)(2.2J/gc)) } }[/math]
[math]\displaystyle{ {t_f} = {27.576°C} }[/math]
Thermal Expansion
Change in temperature through heat transfer can change the matter to change in shape, area, and volume. When a substance is heated, the kinetic energy of its molecules increases. Then the molecules begin moving more and average separation between molecules become larger, and thus volume of the substance changes. The degree of expansion divided by the change in temperature is called the material's coefficient of thermal expansion and generally varies with temperature and the object and state of matter of the object.
| Material | Fractional expansion per degree C x10^-6 | Fractional expansion per degree F x10^-6 |
|---|---|---|
| Glass, ordinary | 9 | 5 |
| Glass, pyrex | 4 | 2.2 |
| Quartz, fused | .59 | .33 |
| Aluminum | 24 | 13 |
| Brass | 19 | 11 |
| Copper | 17 | 9.4 |
| Iron | 12 | 6.7 |
| Steel | 13 | 7.2 |
| Platinum | 9 | 5 |
| Tungsten | 4.3 | 2.4 |
| Gold | 14 | 7.8 |
| Silver | 18 | 10 |
History
Early explanations of heat were thoroughly confused with explanations of combustion. J. J. Becher and George Ernst Stahl introduced the phlogiston theory of combustion in the 17th century, and phlogiston was thought to be the substance of heat.
In late 18th century, Antoine Lavoisier argued that phlogiston theory is inconsistent and introduced Caloric theory. The suggested explanation was that when an object was heated, an invisible fluid called “caloric” was added to the object. Hot objects contained more caloric than cold objects. Caloric theory was popularly accepted until mid 19th century.
Followed by introduction of caloric theory, Count Rumford found that boring a cannon repeatedly does not result in heat producing ability, and therefore caloric is not lost. This proposed that caloric might not be a substance though the experimental ambiguity in his experiment were widely considered controversial.
In mid 19th century, Rudolf Clausius showed that Rumford's argument and Caloric theory can be agreeable if the caloric theory include the principle of conservation of energy in place of its original principle of conservation of heat. By doing so, the caloric theory was able to be evolved into modern thermodynamics where heat may be put as a thermal energy which is equivalent to kinetic energy of some particles of the substance.

References
http://hyperphysics.phy-astr.gsu.edu/hbase/tables/thexp.html
http://hyperphysics.phy-astr.gsu.edu/hbase/thermo/temper.html
http://teachers.oregon.k12.wi.us/mahr/assignments/thermal_energyvs_temp.pdf