Crystalline Structures: Difference between revisions
No edit summary |
|||
| Line 1: | Line 1: | ||
==Bravais lattices== | ==Bravais lattices== | ||
Bravais Lattices are the basic patterns that describe how points can be arranged in space to form a crystal lattice. Named after Auguste Bravais, who studied them in 1848, there are 14 unique Bravais lattices, each representing a distinct way to repeat a pattern in three dimensions. These lattices are grouped into seven crystal systems: cubic, tetragonal, orthorhombic, hexagonal, trigonal, monoclinic, and triclinic. Each lattice is defined by its symmetry and how it repeats in space, forming the backbone of all crystalline materials. Understanding Bravais lattices is important because they help explain how atoms are arranged in crystals and how this arrangement affects a material's properties like strength, conductivity, and optical behavior. | Bravais Lattices are the basic patterns that describe how points can be arranged in space to form a crystal lattice. Named after Auguste Bravais, who studied them in 1848, there are 14 unique Bravais lattices, each representing a distinct way to repeat a pattern in three dimensions. These lattices are grouped into seven crystal systems: cubic, tetragonal, orthorhombic, hexagonal, trigonal, monoclinic, and triclinic. Each lattice is defined by its symmetry and how it repeats in space, forming the backbone of all crystalline materials. Understanding Bravais lattices is important because they help explain how atoms are arranged in crystals and how this arrangement affects a material's properties like strength, conductivity, and optical behavior. | ||
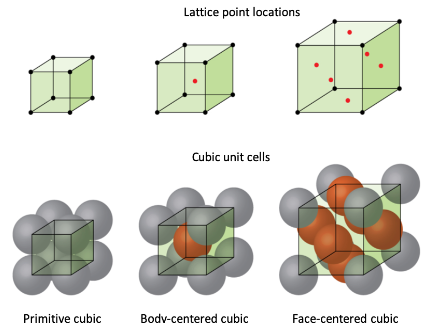
The main types of configurations that a Bravais Lattice can take is: Privative, Body-Centered Cubic, and Face-Centered cubic | |||
[[File:FCCBCC.png]] | [[File:FCCBCC.png]] | ||
===Body-Centered Cubic=== | ====Primitive (Simple Cubic):==== | ||
In this configuration, atoms are located only at the corners of the unit cell. Each corner atom is shared with adjacent unit cells, resulting in one net atom per unit cell. The packing efficiency is low, making this arrangement relatively rare in nature. | |||
Example: Polonium, Caesium Chloride | |||
====Body-Centered Cubic==== | |||
In addition to the corner atoms, there is a single atom located at the center of the unit cell. This arrangement increases the packing efficiency compared to the primitive structure but still leaves some empty space within the lattice. | |||
Example: Iron, Tungsten, Potassium (K) | |||
===Face-Centered Cubic=== | ===Face-Centered Cubic==== | ||
Atoms are present at each corner and the centers of all the cube faces. This configuration has the highest packing efficiency among the cubic structures (74%), leading to dense and stable crystal arrangements. | |||
Example: Aluminum, Copper, Gold, Salt | |||
==Impurities== | ==Impurities== | ||
Revision as of 21:32, 6 December 2024
Bravais lattices
Bravais Lattices are the basic patterns that describe how points can be arranged in space to form a crystal lattice. Named after Auguste Bravais, who studied them in 1848, there are 14 unique Bravais lattices, each representing a distinct way to repeat a pattern in three dimensions. These lattices are grouped into seven crystal systems: cubic, tetragonal, orthorhombic, hexagonal, trigonal, monoclinic, and triclinic. Each lattice is defined by its symmetry and how it repeats in space, forming the backbone of all crystalline materials. Understanding Bravais lattices is important because they help explain how atoms are arranged in crystals and how this arrangement affects a material's properties like strength, conductivity, and optical behavior.
The main types of configurations that a Bravais Lattice can take is: Privative, Body-Centered Cubic, and Face-Centered cubic
Primitive (Simple Cubic):
In this configuration, atoms are located only at the corners of the unit cell. Each corner atom is shared with adjacent unit cells, resulting in one net atom per unit cell. The packing efficiency is low, making this arrangement relatively rare in nature.
Example: Polonium, Caesium Chloride
Body-Centered Cubic
In addition to the corner atoms, there is a single atom located at the center of the unit cell. This arrangement increases the packing efficiency compared to the primitive structure but still leaves some empty space within the lattice.
Example: Iron, Tungsten, Potassium (K)
Face-Centered Cubic=
Atoms are present at each corner and the centers of all the cube faces. This configuration has the highest packing efficiency among the cubic structures (74%), leading to dense and stable crystal arrangements.
Example: Aluminum, Copper, Gold, Salt