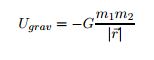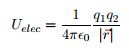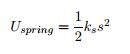The Energy Principle: Difference between revisions
| Line 19: | Line 19: | ||
EQ 2: <math>{∆E} = {∆K + ∆E_{Rest} + ∆U}</math> - the different types of energy that can be associated with a given particle in a system. Not all have to be present. | EQ 2: <math>{∆E} = {∆K + ∆E_{Rest} + ∆U + ∆E_{Thermal}}</math> - the different types of energy that can be associated with a given particle in a system. Not all have to be present. | ||
EQ 3: <math> E_{Rest}=mc^2 </math> - Rest Energy, where <b>m</b> is the mass and <b>c</b> is the speed of light. | EQ 3: <math> E_{Rest}=mc^2 </math> - Rest Energy, where <b>m</b> is the mass and <b>c</b> is the speed of light. | ||
Revision as of 16:37, 1 December 2015
Claimed by lakshitha
The energy principle is a broad equation that can be used to describe the changes in the different types of energy and any work that acts on a system or the transfer of heat into a system. There are many different types of energy, some of which include Kinetic Energy, Potential Energy, Chemical Energy, Rest Energy, Thermal Energy, etc.
The Main Idea
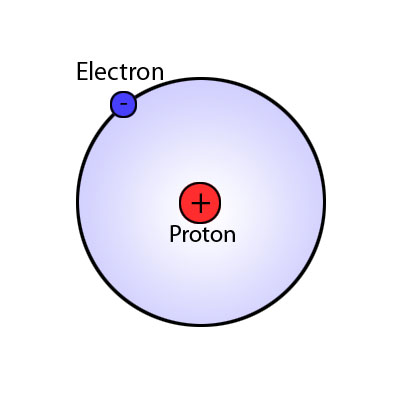
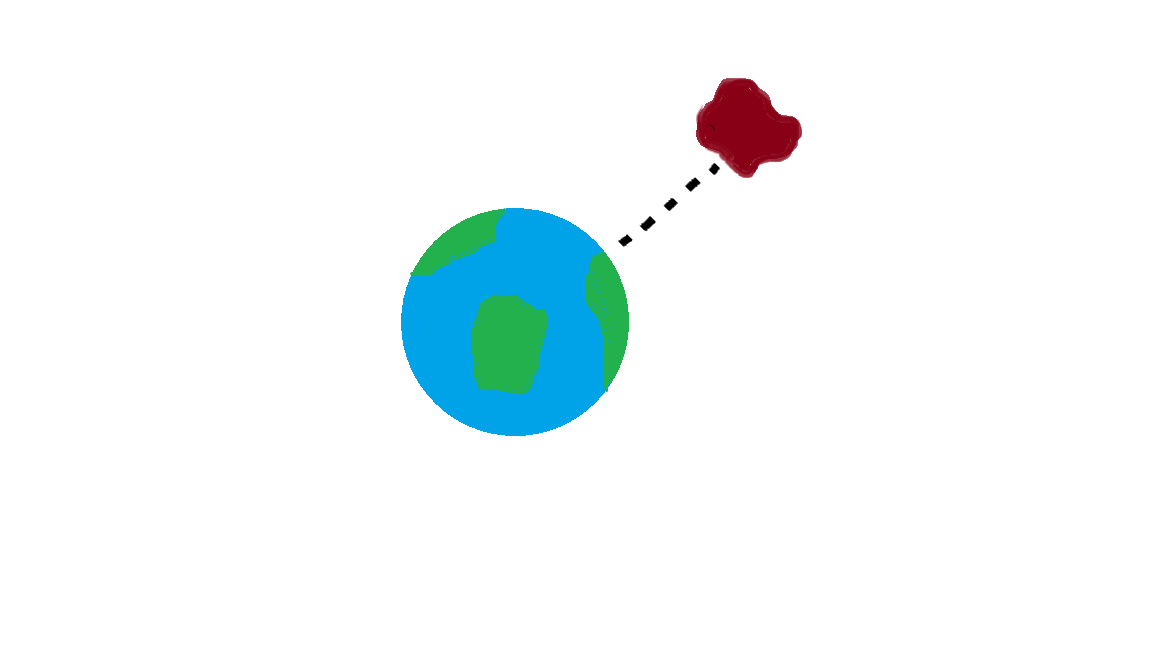
There are many ways to use the energy principle; most of the problems you will encounter will have no work acting on the system and no transfer of heat, thus Esys,initial = Esys,final. Other problems will involve only work acting on the system and no heat. It's important to identify what your system is before making any assumptions about its energy and whether there is any transfer of heat or any work acting on it. It's also useful to note that all objects have rest energy and a object has kinetic energy if it is moving; but, since rest energy is only changed when there is a change in mass of the object, it can usually be disregarded in any calculations. There are also different types of potential energy: Spring Potential Energy, Gravitational Potential Energy, Electric Potential Energy, and Gravitational Potential Energy near the surface of the Earth. Potentially Energy is only included if there is more than one object in your choice of system. For example, a system consisting of one proton and one electron would have Electric Potential Energy. But, a system of an asteroid and Earth would have Gravitational Potential Energy.
For any multi-particle system, you have to take into account the potential energy between each of the objects.
Mathematical Models
NOTE: These are a lot of equations, but don't get overwhelmed. You simply have to pick and choose which ones are necessary for the problem given; examples are shown below. For in-depth explanations on each type of energy, look at the associated pages on the Main Page or click on the links in the 'See also' section below.
The Energy Principle
EQ 1: [math]\displaystyle{ {∆E} = {Q + W} }[/math] where [math]\displaystyle{ {Q} }[/math] is heat and [math]\displaystyle{ {W} }[/math] is the amount of work acting on the system.
EQ 2: [math]\displaystyle{ {∆E} = {∆K + ∆E_{Rest} + ∆U + ∆E_{Thermal}} }[/math] - the different types of energy that can be associated with a given particle in a system. Not all have to be present.
EQ 3: [math]\displaystyle{ E_{Rest}=mc^2 }[/math] - Rest Energy, where m is the mass and c is the speed of light.
EQ 4: [math]\displaystyle{ K=\frac{1}{2}mv² }[/math] - Kinetic Energy, where m is the mass and v is the velocity (for speeds less than the speed of light).
Potential Energy Equations:
A Computational Model
How do we visualize or predict using this topic. Consider embedding some vpython code here Teach hands-on with GlowScript
Examples
Be sure to show all steps in your solution and include diagrams whenever possible
Simple
Middling
Difficult
Connectedness
The Energy Principle can be used for a variety of situations; the fact that it can tell us something about the work acting on a system with only knowing about what energies are present (and vice versa) is what makes this such a fundamental principle. The energy principle is also used to describe the conservation of energy, which is something I find pretty interesting. Energy doesn't just disappear, it's simply converted into a different form. This topic has a huge connection to my major, Biology, because chemical energy (obtained from food) is necessary for a healthy and active body.
History
Thermodynamics was brought up as a science in the 18th and 19th centuries. However, it was first brought up by Galilei, who introduced the concept of temperature and invented the first thermometer. G. Black first introduced the word 'thermodynamics'. Later, G. Wilke introduced another unit of measurement known as the calorie that measures heat. The idea of thermodynamics was brought up by Nicolas Leonard Sadi Carnot. He is often known as "the father of thermodynamics". It all began with the development of the steam engine during the Industrial Revolution. He devised an ideal cycle of operation. During his observations and experimentations, he had the incorrect notion that heat is conserved, however he was able to lay down theorems that led to the development of thermodynamics. In the 20th century, the science of thermodynamics became a conventional term and a basic division of physics. Thermodynamics dealt with the study of general properties of physical systems under equilibrium and the conditions necessary to obtain equilibrium.
See also
Are there related topics or categories in this wiki resource for the curious reader to explore? How does this topic fit into that context?
Further reading
Books, Articles or other print media on this topic
External links
Internet resources on this topic
References
https://www.grc.nasa.gov/www/k-12/airplane/thermo0.html http://hyperphysics.phy-astr.gsu.edu/hbase/thermo/thereq.html https://www.grc.nasa.gov/www/k-12/airplane/thermo2.html http://www.phys.nthu.edu.tw/~thschang/notes/GP21.pdf http://www.eoearth.org/view/article/153532/