Electronic Energy Levels and Photons: Difference between revisions
No edit summary |
No edit summary |
||
| Line 5: | Line 5: | ||
Electrons can be excited by absorbing energy from photons. Electrons can only be excited to certain electronic energy levels. Each electronic energy level is a number that represents the sum of the kinetic and potential energy (K+U). Because electrons are only stable at those energy levels, an electron can only absorb certain energies from photons. After the electron is excited, it drops down and releases a photon. It can drop to any energy level below it, and thus the resulting photons can be of several energies. | Electrons can be excited by absorbing energy from photons. Electrons can only be excited to certain electronic energy levels. Each electronic energy level is a number that represents the sum of the kinetic and potential energy (K+U). Because electrons are only stable at those energy levels, an electron can only absorb certain energies from photons. After the electron is excited, it drops down and releases a photon. It can drop to any energy level below it, and thus the resulting photons can be of several energies. | ||
[[File:hydrogen. | [[File:hydrogen.png|200px|thumb|left|Hydrogen and its energy levels]] | ||
===A Mathematical Model=== | ===A Mathematical Model=== | ||
Revision as of 00:26, 2 December 2015
Short Description of Topic
The Main Idea
Electrons can be excited by absorbing energy from photons. Electrons can only be excited to certain electronic energy levels. Each electronic energy level is a number that represents the sum of the kinetic and potential energy (K+U). Because electrons are only stable at those energy levels, an electron can only absorb certain energies from photons. After the electron is excited, it drops down and releases a photon. It can drop to any energy level below it, and thus the resulting photons can be of several energies.
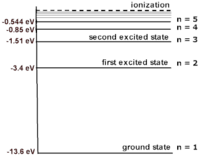
A Mathematical Model
For example, the electronic energy levels for a hydrogen atom can be modeled by the equation: [math]\displaystyle{ {\frac{-13.6}{N^2}} = {K+U} }[/math] where p is the momentum of the system and F is the net force from the surroundings.
A Computational Model
How do we visualize or predict using this topic. Consider embedding some vpython code here Teach hands-on with GlowScript
Examples
Be sure to show all steps in your solution and include diagrams whenever possible
Simple
Middling
Difficult
Connectedness
- How is this topic connected to something that you are interested in?
- How is it connected to your major?
- Is there an interesting industrial application?
History
Put this idea in historical context. Give the reader the Who, What, When, Where, and Why.
See also
Are there related topics or categories in this wiki resource for the curious reader to explore? How does this topic fit into that context?
Further reading
Books, Articles or other print media on this topic
External links
Internet resources on this topic
References
This section contains the the references you used while writing this page