Wave-Particle Duality: Difference between revisions
No edit summary |
No edit summary |
||
| Line 22: | Line 22: | ||
===A Mathematical Model=== | ===A Mathematical Model=== | ||
Now that we can treat these particles at the quantum level as waves, we can use many different equations from wave mechanics to describe their behavior. One of the most important equations in dealing with wave like properties of these quantum systems and particles is the [https://en.wikipedia.org/wiki/Schr%C3%B6dinger_equation Schrödinger equation]. The Schrödinger equation is the analog of [https://en.wikipedia.org/wiki/Newton%27s_laws_of_motion Newton's second law] ('''F''' = ''m'''''a''') in quantum mechanics, and describes the wave function over time of a | Now that we can treat these particles at the quantum level as waves, we can use many different equations from wave mechanics to describe their behavior. One of the most important equations in dealing with wave like properties of these quantum systems and particles is the [https://en.wikipedia.org/wiki/Schr%C3%B6dinger_equation Schrödinger equation]. The Schrödinger equation is the analog of [https://en.wikipedia.org/wiki/Newton%27s_laws_of_motion Newton's second law] ('''F''' = ''m'''''a''') in quantum mechanics, and describes the wave function over time of a system such as a particle moving in a magnetic field. But rather than a simple linear equation, the Schrödinger equation is a linear partial differential equation: | ||
<math>i \hbar \frac{\partial}{\partial t}\Psi(\mathbf{r},t) = \hat H \Psi(\mathbf{r},t)</math> | |||
Revision as of 22:45, 2 December 2015
Really, vservera3? Really? -the person you stole this page from -what are you talking about... I wrote all this
Wave-particle duality is the concept that states every elementary particle behaves like both a wave and a particle.
The Main Idea
In the 1920s, a French physicist named Louis de Broglie suggested that all matter has wave-like properties. This conclusion was largely the result of two landmark experiments that contradicted each other in almost every way. The first experiment was Thomas Young's double slit experiment, which showed light behaved like a wave. The second experiment was by Albert Einstein, who showed, through his research on the photoelectric effect, that light was made up of discrete packets of energy called photons -- which meant that light also behaved as a particle. This contradiction sent the world of physics as humans knew it into panic.
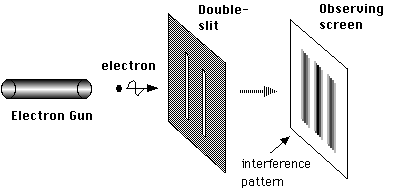
Double slit experiment
The double slit experiment is a deceptively simple experiment that was originally conducted by Thomas Young in the 17th century. In the experiment, Young simply sent a beam of light through two slits and observed the pattern on the surface behind the slits. What he saw was an interference pattern that only could have been present if waves were what went inside two slits. The bright spots occur where the amplitudes of the two waves match (both waves are at their peaks) and the dark spots occur when one wave is at its maximum amplitude and the other is at its minimum.

Photoelectric effect
It was known that when light struck a metal, electrons were liberated from the surface. The intuition was that increasing the intensity of light (shining more light) would liberate more electrons. Albert Einstein found something interesting, though. Varying intensity of light had no effect on how many electrons were liberated. Rather, the frequency of the light determined how many electrons, if any, would be freed. Furthermore, the original theory was that the electrons that would be freed was continuous -- even the smallest amount of light would free some electrons. In fact, this was not the case. Einstein found that there was a minimum threshold frequency that must have been present in order to release electrons at all. This implied there was a minimum amount of energy, or quantum involved in the interaction. This pointed to the fact that light in fact behaved as particles (called photons) which were packets of these quantum energies. This directly conflicted with the double slit experiment.

PhET Simulation for Photoelectric effect
A Mathematical Model
Now that we can treat these particles at the quantum level as waves, we can use many different equations from wave mechanics to describe their behavior. One of the most important equations in dealing with wave like properties of these quantum systems and particles is the Schrödinger equation. The Schrödinger equation is the analog of Newton's second law (F = ma) in quantum mechanics, and describes the wave function over time of a system such as a particle moving in a magnetic field. But rather than a simple linear equation, the Schrödinger equation is a linear partial differential equation:
[math]\displaystyle{ i \hbar \frac{\partial}{\partial t}\Psi(\mathbf{r},t) = \hat H \Psi(\mathbf{r},t) }[/math]
A Computational Model
How do we visualize or predict using this topic. Consider embedding some vpython code here Teach hands-on with GlowScript
Examples
Be sure to show all steps in your solution and include diagrams whenever possible
Simple
Middling
Difficult
Connectedness
- How is this topic connected to something that you are interested in?
- How is it connected to your major?
- Is there an interesting industrial application?
History
Put this idea in historical context. Give the reader the Who, What, When, Where, and Why.
See also
Are there related topics or categories in this wiki resource for the curious reader to explore? How does this topic fit into that context?
Further reading
Books, Articles or other print media on this topic
External links
Internet resources on this topic
References
This section contains the the references you used while writing this page