Spontaneous Photon Emission: Difference between revisions
No edit summary |
No edit summary |
||
| Line 13: | Line 13: | ||
Above you'll see the emissions spectrums for hydrogen and krypton respectively. As you can see, krypton has a much wider range of potential photon emissions, largely because its atoms are far more complex than those of a more simple element like hydrogen. | Above you'll see the emissions spectrums for hydrogen and krypton respectively. As you can see, krypton has a much wider range of potential photon emissions, largely because its atoms are far more complex than those of a more simple element like hydrogen. | ||
=== | ===Mathematical Model=== | ||
The primary basis for our understanding of the process of spontaneous photon emission comes from the law of conservation of energy, <math>{E}_{final} = {E}_{initial}</math>. | The primary basis for our understanding of the process of spontaneous photon emission comes from the law of conservation of energy, <math>{E}_{final} = {E}_{initial}</math>. | ||
Revision as of 22:20, 3 December 2015
page in progress by kylerasmussen44
Spontaneous photon emission is a process that occurs when an atom or other quantum system goes down an energy level, and releases a photon. This process is often incited by the absorption of a particle whose energy causes an atom to increase its energy level, and enter an excited state; in this case, spontaneous photon emission would move the atom to a lower energy level, closer to its initial state. This process results in the production of light, and has been instrumental in many inventions, such as fluorescent lights, television displays and light emitting diodes.
The Main Idea
If an atom is in an excited state, meaning that its current energy level is higher than the minimum energy level, or ground state, it may undergo the process of spontaneous photon emission, decreasing its energy level to one closer to the ground state. Through this process, an atom will decrease its energy level, and emit a photon with energy equal to the difference in energy between the two energy levels.
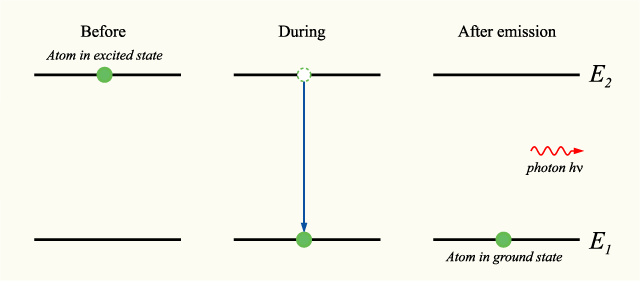 In accordance with the law of conservation of energy, if we chose a system including both the photon and the atom, this process will feature no net energy change.
The collection of photon emissions for an atom's transition from a higher to a lower state is called an emission spectrum. For any given atom in an excited state, there typically exists a wide range of potential photon emissions, and these emissions vary greatly between different elements.
In accordance with the law of conservation of energy, if we chose a system including both the photon and the atom, this process will feature no net energy change.
The collection of photon emissions for an atom's transition from a higher to a lower state is called an emission spectrum. For any given atom in an excited state, there typically exists a wide range of potential photon emissions, and these emissions vary greatly between different elements.


Above you'll see the emissions spectrums for hydrogen and krypton respectively. As you can see, krypton has a much wider range of potential photon emissions, largely because its atoms are far more complex than those of a more simple element like hydrogen.
Mathematical Model
The primary basis for our understanding of the process of spontaneous photon emission comes from the law of conservation of energy, [math]\displaystyle{ {E}_{final} = {E}_{initial} }[/math].
When an atom decreases its energy level, as they do during spontaneous photon emission, this energy cannot be lost, in accordance with the law of conservation of energy. Instead, it is simply converted into another form, in this case, the kinetic energy of a photon. Because this process only involves two different particles, the atom and the photon, the law of conservation of energy also allows us to know that the change in the energy of the atom, is equal and opposite the change in the energy of the photon, [math]\displaystyle{ {Δ}{E}_{atom} = {-}{Δ}{E}_{photon} }[/math].
A Computational Model
How do we visualize or predict using this topic. Consider embedding some vpython code here Teach hands-on with GlowScript
Examples
Be sure to show all steps in your solution and include diagrams whenever possible
Simple
Middling
Difficult
Connectedness
- How is this topic connected to something that you are interested in?
- How is it connected to your major?
- Is there an interesting industrial application?
History
Put this idea in historical context. Give the reader the Who, What, When, Where, and Why.
See also
Are there related topics or categories in this wiki resource for the curious reader to explore? How does this topic fit into that context?
Further reading
Books, Articles or other print media on this topic
External links
References
This section contains the the references you used while writing this page