Nuclear Fission: Difference between revisions
| Line 4: | Line 4: | ||
==The Main Idea== | ==The Main Idea== | ||
Nuclear fission is the process of the nucleus of an atom splitting into multiple smaller parts and, in doing so, releasing a quantity of energy. This process can happen naturally in the form of radioactive decay or in a | Nuclear fission is the process of the nucleus of an atom splitting into multiple smaller parts and, in doing so, releasing a quantity of energy. This process can happen naturally in the form of radioactive decay or in a man-made nuclear reaction. Radioactive decay is very uncommon amongst most large molecules but does happen naturally for Uranium-235 and Plutonium-239, both of which are isotopes. Uranium-235 fissions when gains extra neurons that trigger its decay. The two substituents that form from the split atom have a mass that is about one tenth of one percent less mass than that of the original atom, this loss of mass is about ten million times larger than the mass changes that occur in chemical reactions that involve rearrangement and do not alter or affect the nucleus. | ||
===Radioactive Decay=== | |||
===Nuclear Reactions=== | |||
Nuclear fission is typically managed to produce a standard and controlled reaction, but when it is not managed it results in a dangerous and uncontrollable release of energy (see atomic bomb). | |||
===A Mathematical Model=== | ===A Mathematical Model=== | ||
Revision as of 17:09, 17 April 2016
This topic is claimed by qmurphy3 NO3 Schatz.
Nuclear fission is the process of splitting up an atom into multiple parts. This occurs spontaneously in the form of radioactive decay. 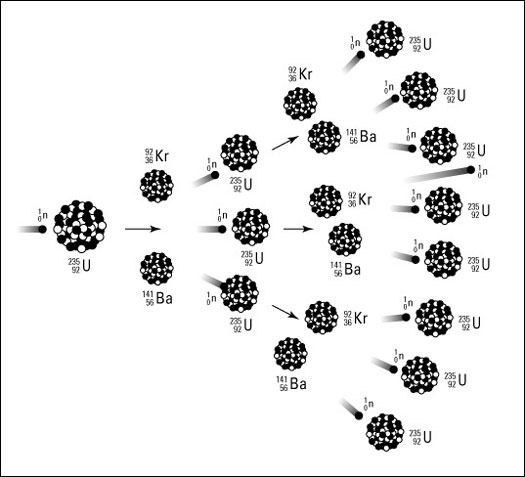
The Main Idea
Nuclear fission is the process of the nucleus of an atom splitting into multiple smaller parts and, in doing so, releasing a quantity of energy. This process can happen naturally in the form of radioactive decay or in a man-made nuclear reaction. Radioactive decay is very uncommon amongst most large molecules but does happen naturally for Uranium-235 and Plutonium-239, both of which are isotopes. Uranium-235 fissions when gains extra neurons that trigger its decay. The two substituents that form from the split atom have a mass that is about one tenth of one percent less mass than that of the original atom, this loss of mass is about ten million times larger than the mass changes that occur in chemical reactions that involve rearrangement and do not alter or affect the nucleus.
Radioactive Decay
Nuclear Reactions
Nuclear fission is typically managed to produce a standard and controlled reaction, but when it is not managed it results in a dangerous and uncontrollable release of energy (see atomic bomb).
A Mathematical Model
The large amount of energy that is released is not lost or destroyed according to the conservation of mass principle, therefore we can use the formula below to better understand the change in mass and energy released during nuclear fission reactions.
Mass of energy released = (E/c^2) = Mass Final- Mass Initial
A Computational Model
Examples
Nuclear power plants like these are found in various countries all over the globe.
Nuclear fission occurs in reactors like this that capture the energy from the reaction.
Connectedness
Nuclear fission is a very interesting topic to me because of its potential for green and renewable energy. After delving deeper into the subject, I was made aware of just how astonishingly large the amount of energy that is produced from the splitting of an atom actually was. I was always aware of what nuclear fission was because of its use in the atomic bomb and the basic explanation in secondary school. This topic however does not directly tie into my major of Biomedical engineering. The results of effective nuclear fission would be energy with which to power some types of biomedical devices as well as the potential to learn more about elements and materials that could be used in nuclear fission and other things. The overall industrial application of nuclear fission is actually quite impressive, it has the potential to be one of the most reliable and consistent forms of power production once the remainder of fossil fuels are depleted. Nuclear fission is a growing form of producing energy and will become an even better alternative once there is an adequate way to dispose of the radioactive waste that it produces.
History
Otto Hahn and his assistant first discovered heavy nuclear fission in 1938. Hahn was a German scientist and won the Nobel Prize in 1945 for discovering chemical proof of nuclear fission. He discovered this by experimentally bombarding Uranium with neurons, the same method that is used today. The bombardment resulted in isotopes on the alkaline metal that they were testing as the sample, it was initially suspected that this was radium but after more thorough testing it was concluded that it was Barium, a product of splitting Uranium.
See also
- Radioactive Decay
- Nuclear Fusion
- Renewable Energy
Further reading
"The Nuclear Fission Process" by Cyriel Wagemans
References
Chabay, Ruth W., and Bruce A. Sherwood. "Chapter 10: Collisions." Matter & Interactions. Fourth Edition ed. Wiley, 2015. 261. Print. http://hyperphysics.phy-astr.gsu.edu/hbase/nucene/fission.html http://ieer.org/resource/factsheets/basics-nuclear-physics-fission/ https://en.wikipedia.org/wiki/Nuclear_fission https://en.wikipedia.org/wiki/Otto_Hahn


