Bohr Model: Difference between revisions
Pearlruparel (talk | contribs) |
m (Typos) |
||
| Line 18: | Line 18: | ||
1) | 1) | ||
|Ltrans,nucleus| = Nh* | |Ltrans,nucleus| = Nh*,rp = Nh* | ||
| Line 50: | Line 50: | ||
[[File: Screen_Shot_2015-12-02_at_8.27.42_PM.png |left|500x500px|thumb|Energy vs Time Graph, [3]]] | [[File: Screen_Shot_2015-12-02_at_8.27.42_PM.png |left|500x500px|thumb|Energy vs Time Graph, [3]]] | ||
Here is a visualization of the Bohr Model, and its graph of Energy (eV), Kinetic Energy, and Potential Energy . This visualization shows how the electrons jump from level to level according to the Bohr Model. There is also | Here is a visualization of the Bohr Model, and its graph of Energy (eV), Kinetic Energy, and Potential Energy. This visualization shows how the electrons jump from level to level according to the Bohr Model. There is also an energy vs distance graph shown which varies according to these levels. | ||
Here is the link to visualization to try out different levels and see the energy graphs accordingly. In order to do this visualization, visit the link given below: http://www.glowscript.org/#/user/matterandinteractions/folder/matterandinteractions/program/08-Bohr-levels | |||
[[File: Screen_Shot_2015-12-02_at_8.27.28_PM.png |center|300x300px|thumb|Energy vs Distance Graph, [4]]] | [[File: Screen_Shot_2015-12-02_at_8.27.28_PM.png |center|300x300px|thumb|Energy vs Distance Graph, [4]]] | ||
| Line 84: | Line 84: | ||
K+U = energy of photon = <math>E(1) - E(3) = {\frac{-13.6 eV}{3^2}} - {\frac{-13.6 eV}{1^2}}</math> = 12.09 Joules | K+U = energy of photon = <math>E(1) - E(3) = {\frac{-13.6 eV}{3^2}} - {\frac{-13.6 eV}{1^2}}</math> = 12.09 Joules | ||
To the right is the graph of as the hydrogen atom goes from N = 3 to N = 1. | To the right is the graph of E as the hydrogen atom goes from N = 3 to N = 1. | ||
| Line 95: | Line 95: | ||
===Difficult=== | ===Difficult=== | ||
Hydrogen has been detected transitioning from the | Hydrogen has been detected transitioning from the 101<sup>st</sup> to the 100<sup>th</sup> energy levels. What is the wavelength of the radiation? Where in the electromagnetic spectrum is this emission? | ||
To solve this problem, we first need to use | |||
Then solve for the | To solve this problem, we first need to use formulas derived from Bohr Model of hydrogen atom. It is <math>E = {\frac{-13.6 eV}{N^2}}</math> | ||
Then solve for the wavelength using formula from Electromagnetic Wave Theory. | |||
[[Image:Screen Shot 2015-12-01 at 8.21.56 PM.png|left|350x350px|]] | [[Image:Screen Shot 2015-12-01 at 8.21.56 PM.png|left|350x350px|]] | ||
| Line 115: | Line 120: | ||
This wavelength falls in the microwave portion of the electromagnetic spectrum. | |||
==Connectedness== | ==Connectedness== | ||
This topic is quite interesting as it is the initial introduction to quantum physics, which I find particularly intriguing. Although, this model has had shortcomings, it is one of the most successful models of its time. It has many features that are used in the actual model for quantum physics. This model also involves coding to show the visualization, which is how it somewhat related to Computer Science. Additionally, there are quite a few applications of the Bohr's Model. Bohr's discovery of the quantum leap is, in many ways, the most shining example of the consequences which Bohr's model of the atom has had for modern society.Bohr's notion that atoms emit light quanta with very specific energies is behind many of the technologies on which we depend our daily lives. Laser technology, which is becoming very popular in today's world depends entirely on principles behind Bohr's model of atom because laser light is produced by quantum leaps. The quantum leaps between the specific energy levels show that light has specific frequency and wavelength which measures time and length precisely. However, due to shortcomings in Bohr's Model we can't completely | This topic is quite interesting as it is the initial introduction to quantum physics, which I find particularly intriguing. Although, this model has had shortcomings, it is one of the most successful models of its time. It has many features that are used in the actual model for quantum physics. This model also involves coding to show the visualization, which is how it somewhat related to Computer Science. Additionally, there are quite a few applications of the Bohr's Model. Bohr's discovery of the quantum leap is, in many ways, the most shining example of the consequences which Bohr's model of the atom has had for modern society. Bohr's notion that atoms emit light quanta with very specific energies is behind many of the technologies on which we depend our daily lives. Laser technology, which is becoming very popular in today's world, depends entirely on principles behind Bohr's model of atom because laser light is produced by quantum leaps. The quantum leaps between the specific energy levels show that light has specific frequency and wavelength which measures time and length precisely. However, due to shortcomings in Bohr's Model, we can't completely utilize these calculations, but the framework is used. | ||
==History== | ==History== | ||
[[Image:bohr.jpg|right|100x100px, [5]]] [5] The first successful model of hydrogen was developed by Bohr in 1913, and incorporated the new ideas of quantum theory. Neils Bohr explained the emission spectra of hydrogen by improving on the Rutherford model of the atom. Bohr’s model improved the classical atomic models of physicists J. J. Thomson and Ernest Rutherford by incorporating quantum theory. While working on his doctoral dissertation at Copenhagen University, Bohr studied physicist Max Planck’s quantum theory of radiation. Then after graduating, Bohr worked in England with Thomson and subsequently with Rutherford to come up with this model. During this time Bohr developed his model of atomic structure. Initially, Rutherford's planetary model predicted a continuous spectrum of light from hydrogen. However, Bohr corrected for this by proposing that the translational angular momentum of the electron can be quantized. Although this model is not entirely correct, it has many features that are and is therefore used in physics. | [[Image:bohr.jpg|right|100x100px, [5]]] [5] The first successful model of hydrogen was developed by Bohr in 1913, and incorporated the new ideas of quantum theory. Neils Bohr explained the emission spectra of hydrogen by improving on the Rutherford model of the atom. Bohr’s model improved the classical atomic models of physicists J. J. Thomson and Ernest Rutherford by incorporating quantum theory. While working on his doctoral dissertation at Copenhagen University, Bohr studied physicist Max Planck’s quantum theory of radiation. Then after graduating, Bohr worked in England with Thomson and subsequently with Rutherford to come up with this model. During this time, Bohr developed his model of atomic structure. Initially, Rutherford's planetary model predicted a continuous spectrum of light from hydrogen. However, Bohr corrected for this by proposing that the translational angular momentum of the electron can be quantized. Although this model is not entirely correct, it has many features that are and is therefore used in physics. | ||
| Line 130: | Line 136: | ||
== Shortcomings of the Bohr Model == | == Shortcomings of the Bohr Model == | ||
The Bohr Model is an important predecessor to the current quantum mechanical models of the atom. However, there are some characteristics of the Bohr model | The Bohr Model is an important predecessor to the current quantum mechanical models of the atom. However, there are some characteristics of the Bohr model that are not entirely correct. The actual quantization rules in a hydrogen atom are much more complex than those assumed in the Bohr Model. The translational angular momentum in ground state (N = 1), is zero, not h, and for the next higher state of N = 2, the z component of translational angular momentum can either be zero or h. Other issues with the Bohr Model include that it violates the Heisenberg Uncertainty Principle because it considers electrons to have both a known radius and orbit. It also makes poor predictions regarding spectra of larger atoms, and does not predict the relative intensities of spectral lines. | ||
== See also == | == See also == | ||
Revision as of 13:01, 26 April 2016
by Pearl Ruparel
This page gives basic information about the Bohr Model and Quantization. It also includes examples using Bohr Model.
Main Idea
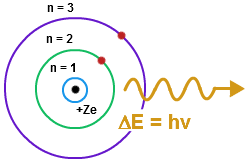
In atomic physics, the Bohr model depicts the atom as a small, positively charged nucleus surrounded by electrons in orbit similar in structure to the solar system. In this model, the neutrons and protons occupy a dense central region (the nucleus), while the electrons orbit the nucleus, like the planets orbit the Sun. This is why the Bohr Model is commonly referred to as the "planetary model" [2].It is taught as an introduction to quantum physics. In the Bohr Model, electrons can only be at certain, different, distances from the proton to which it is bound. Energy is quantized as explained by the Bohr Model. This means that only orbits with certain radii are allowed, while orbits in between simply don't exist. These levels are knows an quantized energy levels and are labeled with integer N known as quantum number. The lowest energy state is generally termed the ground state. The states with successively more energy than the ground state are called the first excited state, the second excited state, and so on. As the electrons become further away from the nucleus, they become larger and have higher energy. Beyond an energy called the ionization potential the single electron of the hydrogen atom is no longer bound to the atom. The Bohr model works well for very simple atoms such as hydrogen (which has 1 electron) but not for more complex atoms. Although the Bohr model is still used today, especially in elementary textbooks, a more complex model known as the quantum mechanical model is the more accurate version of the Bohr Model and used universally.
A Mathematical Model
This model gives us the formula for the radius derived from translational angular momentum.
|Ltrans,nucleus| = Nh* where N = 1,2,3
Note: h* is used when is not the actual notation for it.
h* = h/2π = 1.05 * 10^-34 J*s
1) |Ltrans,nucleus| = Nh*,rp = Nh*
2)
From curving motion:
[math]\displaystyle{ |Fperpendicular| = {\frac{|p| |v|}{r}} = {\frac{e^2}{4π ε0 r^2}} }[/math]
3)
Substitute in Bohr's Condition:
[math]\displaystyle{ {\frac{N^2 h*^2}{mr^3}} = {\frac{e^2}{4π ε0 r^2}} }[/math]
4) Solve for the Radius [math]\displaystyle{ r = {\frac{4π ε0 h*^2}{me^2}} *N^2 }[/math] where N = 1,2,3
Thus Bohr's Model derives equation for the radius.
Additionally, the formula for energy of hydrogen atom of different levels is also derived from this model.
E = K + Uelectric
1) [math]\displaystyle{ E = {\frac{mv^2}{2}} - {\frac{{\frac{1}{2}}*{\frac{1}{4π ε0}}*{\frac{me^2}{h*}}}{N^2}} }[/math]
2) [math]\displaystyle{ E = {\frac{13.6 eV}{N^2}} }[/math] where N = 1,2,3
A Computational Model

Here is a visualization of the Bohr Model, and its graph of Energy (eV), Kinetic Energy, and Potential Energy. This visualization shows how the electrons jump from level to level according to the Bohr Model. There is also an energy vs distance graph shown which varies according to these levels. Here is the link to visualization to try out different levels and see the energy graphs accordingly. In order to do this visualization, visit the link given below: http://www.glowscript.org/#/user/matterandinteractions/folder/matterandinteractions/program/08-Bohr-levels

Examples
Simple
How much energy in electron volts is required to ionize a hydrogen atom, if initially the atom is in the state N = 3? Here we can use the formula for the hydrogen atom which is
1) [math]\displaystyle{ E = {\frac{-13.6 eV}{N^2}} }[/math]
2) [math]\displaystyle{ ={\frac{-13.6 eV}{3^2}} }[/math] = -1.51 Joules
Middling
[6]A hydrogen atom is in state N = 3, where N = 1 is the lowest energy state. What is K+U in electron volts for this atomic hydrogen energy state?
![250x250px, [6]](/images/c/c1/Screen_Shot_2015-12-03_at_9.37.48_PM.png)
1)
[math]\displaystyle{ E(3) = {\frac{-13.6 eV}{3^2}} }[/math] = -1.51 Joules
2) [math]\displaystyle{ E(1) = {\frac{-13.6 eV}{1^2}} }[/math] = -13.6 Joules
3) K+U = energy of photon = [math]\displaystyle{ E(1) - E(3) = {\frac{-13.6 eV}{3^2}} - {\frac{-13.6 eV}{1^2}} }[/math] = 12.09 Joules
To the right is the graph of E as the hydrogen atom goes from N = 3 to N = 1.
Difficult
Hydrogen has been detected transitioning from the 101st to the 100th energy levels. What is the wavelength of the radiation? Where in the electromagnetic spectrum is this emission?
To solve this problem, we first need to use formulas derived from Bohr Model of hydrogen atom. It is [math]\displaystyle{ E = {\frac{-13.6 eV}{N^2}} }[/math]
Then solve for the wavelength using formula from Electromagnetic Wave Theory.
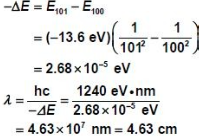
This wavelength falls in the microwave portion of the electromagnetic spectrum.
Connectedness
This topic is quite interesting as it is the initial introduction to quantum physics, which I find particularly intriguing. Although, this model has had shortcomings, it is one of the most successful models of its time. It has many features that are used in the actual model for quantum physics. This model also involves coding to show the visualization, which is how it somewhat related to Computer Science. Additionally, there are quite a few applications of the Bohr's Model. Bohr's discovery of the quantum leap is, in many ways, the most shining example of the consequences which Bohr's model of the atom has had for modern society. Bohr's notion that atoms emit light quanta with very specific energies is behind many of the technologies on which we depend our daily lives. Laser technology, which is becoming very popular in today's world, depends entirely on principles behind Bohr's model of atom because laser light is produced by quantum leaps. The quantum leaps between the specific energy levels show that light has specific frequency and wavelength which measures time and length precisely. However, due to shortcomings in Bohr's Model, we can't completely utilize these calculations, but the framework is used.
History
![100x100px, [5]](/images/e/ee/Bohr.jpg)
[5] The first successful model of hydrogen was developed by Bohr in 1913, and incorporated the new ideas of quantum theory. Neils Bohr explained the emission spectra of hydrogen by improving on the Rutherford model of the atom. Bohr’s model improved the classical atomic models of physicists J. J. Thomson and Ernest Rutherford by incorporating quantum theory. While working on his doctoral dissertation at Copenhagen University, Bohr studied physicist Max Planck’s quantum theory of radiation. Then after graduating, Bohr worked in England with Thomson and subsequently with Rutherford to come up with this model. During this time, Bohr developed his model of atomic structure. Initially, Rutherford's planetary model predicted a continuous spectrum of light from hydrogen. However, Bohr corrected for this by proposing that the translational angular momentum of the electron can be quantized. Although this model is not entirely correct, it has many features that are and is therefore used in physics.
Shortcomings of the Bohr Model
The Bohr Model is an important predecessor to the current quantum mechanical models of the atom. However, there are some characteristics of the Bohr model that are not entirely correct. The actual quantization rules in a hydrogen atom are much more complex than those assumed in the Bohr Model. The translational angular momentum in ground state (N = 1), is zero, not h, and for the next higher state of N = 2, the z component of translational angular momentum can either be zero or h. Other issues with the Bohr Model include that it violates the Heisenberg Uncertainty Principle because it considers electrons to have both a known radius and orbit. It also makes poor predictions regarding spectra of larger atoms, and does not predict the relative intensities of spectral lines.
See also
Further reading
Matter and Interactions I Modern Mechanics 4th Edition Chapter 11.10
External links
https://en.wikipedia.org/wiki/Bohr_model
https://en.wikipedia.org/wiki/Quantization_(physics)
Videos:
https://www.youtube.com/watch?v=nVW1zDPPZGM
Simulation of Bohr Model:
https://phet.colorado.edu/en/simulation/legacy/hydrogen-atom
References
This section contains the the references you used while writing this page