Quantum Properties of Light: Difference between revisions
| Line 46: | Line 46: | ||
<math>p = \frac{h\nu}{c} = \frac{h}{\lambda}</math> | <math>p = \frac{h\nu}{c} = \frac{h}{\lambda}</math> | ||
The last step, is simply to solve for <math>\lambda</math>. This step is left as an exercise to the reader. | |||
===A Computational Model=== | ===A Computational Model=== | ||
Revision as of 20:10, 23 April 2022
Giovanny Espitia, Spring 2022
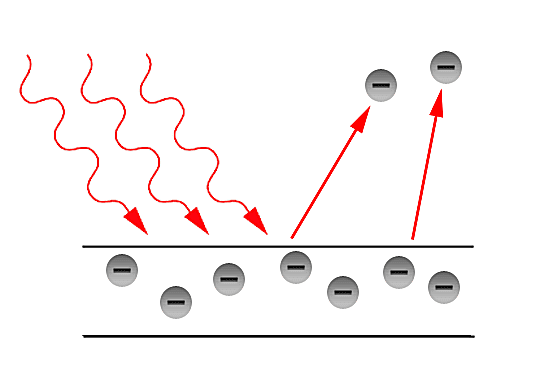
Why do we need a quantum interpretation?
In the latter part of the 19th century, the classical description of light that had prevailed since the 17th century, began to fail in its explanatory power for the ever more precise measurements and phenomena being performed and observed in laboratories. The prime example of such failure is the so - called Ultraviolet Catastrophe. This term was first used by Paul Ehrenfest to refer to Rayleigh - Jeans law ability to predict experimental results below energies of [math]\displaystyle{ 10^{5} }[/math] and its sudden unexplained divergence at energies close to the ultraviolet range in the electromagnetic spectrum. Such discrepancies called for a more fundamental description of light. Fortunately, in the year 1900, Max Planck through his introduction of an ad - hoc assumption, was able to correctly explained the Black - body radiation problem. Planck later referred to such ad - hoc assumption as an "act of desperation". Moreover, in 1905, Einstein further validated the assumption made by Planck in his solution for the Black - body problem. The assumption was to treat light as if it came in discrete - packets later referred to as "Quanta". With such assumption, Einstein made further progress in explaining past experiments such as the Photoelectric effect first observed by Hertz in the latter part of the 19th century.
The Main Idea
Einstein, Planck, and Compton showed the explanatory power of giving light a discrete or quantum interpretation. In his theory of the photoelectric effect, Einstein showed that the energy [math]\displaystyle{ E }[/math] of a photon is simply the product [math]\displaystyle{ h\nu }[/math], where [math]\displaystyle{ h }[/math] is Planck’s constant and [math]\displaystyle{ \nu }[/math] is the frequency of the photon. As mentioned before, such treatment was allowed for the understanding of problems such as the Black - body, the Photoelectrons, and the scattering of electrons upon the contact of high energy electromagnetic radiation and light.
Mathematical Model
The energy of a photon
[math]\displaystyle{ E = h\nu }[/math]
Where:
- [math]\displaystyle{ h = 6.626*10^{-34} }[/math]
- [math]\displaystyle{ \nu = \frac{c}{\lambda} }[/math]
Here, [math]\displaystyle{ c }[/math] is the speed of light and [math]\displaystyle{ \lambda }[/math] is the wavelength of the photon.
Frequency - Wavelength Relationship:
[math]\displaystyle{ c = \lambda\nu }[/math]
Linear momentum of a photon
In the classical perspective, a photon should not carry any momentum since it is a massless boson. However, Einstein showed us through the following relationship that the former statement is not true.
[math]\displaystyle{ E = cp }[/math]
Where [math]\displaystyle{ c }[/math] is the speed of light and [math]\displaystyle{ p }[/math] is the momentum of the photon.
Therefore and according to the energy of a photon expression introduced in the previous subsection
[math]\displaystyle{ E = h\nu = cp }[/math]
Solving for [math]\displaystyle{ p }[/math],
[math]\displaystyle{ p = \frac{h\nu}{c} }[/math]
Interestingly, if we rearrange the above expression in terms of wavelength, we arrive at the same result as De Broglie for the wavelength of a matter wave. This is done as follows:
[math]\displaystyle{ p = \frac{h\nu}{c} }[/math]
Using the frequency - wavelength relationship introduced in a previous section, we make the appropriate substitution. Namely,
[math]\displaystyle{ p = \frac{h\nu}{c} = \frac{h}{\lambda} }[/math]
The last step, is simply to solve for [math]\displaystyle{ \lambda }[/math]. This step is left as an exercise to the reader.
A Computational Model
Hyper physics has a program that allows users to input wavelength, frequency, or energy and find out the other two values. http://hyperphysics.phy-astr.gsu.edu/hbase/mod2.html#c3
Examples
Be sure to show all steps in your solution and include diagrams whenever possible
Simple
If a proton starts at frequency v, and its frequency increases, does its energy increase or decrease?
Its energy increases because E and v have a positive linear correlation. E=hv ?=h2v 2E=h2v
Middling
What is the energy of a single photon of orange light?
Orange light has a radiation frequency of 435 THz.
E=hv
E=( 435*10^12)(6.626*10^-34)
E=2.88*10^-18 Joules
Difficult
Connectedness
This relation allows us to understand the photoelectric effect (why some metals emit electrons when light is shined on them) and Planck's Law of Black Body Radiation.
Every day in the biomedical device industry, new forms of treatment for cancer are being used. Proton emission therapy is a current method. If photons were ever used to combat disease, Planck's Relation could help engineers to calculate their energy or radiation frequency and wavelength in order to adjust these values to get the ones desired.
History
Max Planck, a German Scientist discovered Planck's Relation. He published his paper in 1900. He was doing research and realized that matter could only emit or absorb radiation at certain discrete energy levels. He found a constant that described the distance between the energy levels.
Put this idea in historical context. Give the reader the Who, What, When, Where, and Why.
See also
Are there related topics or categories in this wiki resource for the curious reader to explore? How does this topic fit into that context?
Further reading
HyperPhysics page on Photoelectric Effect: http://hyperphysics.phy-astr.gsu.edu/hbase/mod2.html#c3
External links
A thorough example of a problem calculating the energy in a mole of photons using Planck's Relation: [1]
References
http://hyperphysics.phy-astr.gsu.edu/hbase/mod2.html#c3 https://www.youtube.com/watch?v=dSUvyERhtO8 This section contains the the references you used while writing this page