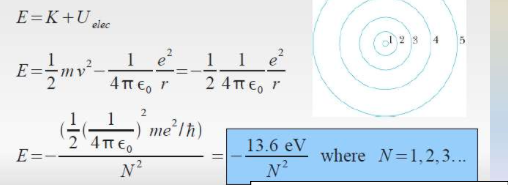Bohr Model: Difference between revisions
Pearlruparel (talk | contribs) No edit summary |
Pearlruparel (talk | contribs) No edit summary |
||
| Line 8: | Line 8: | ||
In atomic physics, the Bohr model depicts the atom as a small, positively charged nucleus surrounded by electrons in orbit similar in structure to the solar system. In the Bohr Model the neutrons and protons occupy a dense central region (the nucleus), while the electrons orbit the nucleus, like the planets orbit the Sun. That is why the Bohr Model is commonly referred tot as the "planetary model".It is taught as an introduction to quantum physics. In the Bohr Model, electrons can only be at certain, discrete, distances from the proton to which it is bound. Energy is quantized as explained by the Bohr Model. This means that only orbits with certain radii are allowed, while orbits in between simply don't exist. These levels are knows an quantized energy levels and are labeled with integer N known as quantum number. The lowest energy state is generally termed the ground state. The states with successively more energy than the ground state are called the first excited state, the second excited state, and so on. As the electrons become further away from the nucleus, they become larger and have higher energy. Beyond an energy called the ionization potential the single electron of the hydrogen atom is no longer bound to the atom. The Bohr model works well for very simple atoms such as hydrogen (which has 1 electron) but not for more complex atoms. Although the Bohr model is still used today, especially in elementary textbooks, a more sophisticated (and complex) model — the quantum mechanical model — is used much more frequently. | In atomic physics, the Bohr model depicts the atom as a small, positively charged nucleus surrounded by electrons in orbit similar in structure to the solar system. In the Bohr Model the neutrons and protons occupy a dense central region (the nucleus), while the electrons orbit the nucleus, like the planets orbit the Sun. That is why the Bohr Model is commonly referred tot as the "planetary model".It is taught as an introduction to quantum physics. In the Bohr Model, electrons can only be at certain, discrete, distances from the proton to which it is bound. Energy is quantized as explained by the Bohr Model. This means that only orbits with certain radii are allowed, while orbits in between simply don't exist. These levels are knows an quantized energy levels and are labeled with integer N known as quantum number. The lowest energy state is generally termed the ground state. The states with successively more energy than the ground state are called the first excited state, the second excited state, and so on. As the electrons become further away from the nucleus, they become larger and have higher energy. Beyond an energy called the ionization potential the single electron of the hydrogen atom is no longer bound to the atom. The Bohr model works well for very simple atoms such as hydrogen (which has 1 electron) but not for more complex atoms. Although the Bohr model is still used today, especially in elementary textbooks, a more sophisticated (and complex) model — the quantum mechanical model — is used much more frequently. | ||
[[File: niels-bohr-model-of-the-hydrogen-atom.png| | [[File: niels-bohr-model-of-the-hydrogen-atom.png|right|thumb|Bohr Model Diagram]] | ||
Revision as of 17:09, 2 December 2015
by Pearl Ruparel
This page gives basic information about the Bohr Model and Quantization. It also includes examples using Bohr Model.
Main Idea
In atomic physics, the Bohr model depicts the atom as a small, positively charged nucleus surrounded by electrons in orbit similar in structure to the solar system. In the Bohr Model the neutrons and protons occupy a dense central region (the nucleus), while the electrons orbit the nucleus, like the planets orbit the Sun. That is why the Bohr Model is commonly referred tot as the "planetary model".It is taught as an introduction to quantum physics. In the Bohr Model, electrons can only be at certain, discrete, distances from the proton to which it is bound. Energy is quantized as explained by the Bohr Model. This means that only orbits with certain radii are allowed, while orbits in between simply don't exist. These levels are knows an quantized energy levels and are labeled with integer N known as quantum number. The lowest energy state is generally termed the ground state. The states with successively more energy than the ground state are called the first excited state, the second excited state, and so on. As the electrons become further away from the nucleus, they become larger and have higher energy. Beyond an energy called the ionization potential the single electron of the hydrogen atom is no longer bound to the atom. The Bohr model works well for very simple atoms such as hydrogen (which has 1 electron) but not for more complex atoms. Although the Bohr model is still used today, especially in elementary textbooks, a more sophisticated (and complex) model — the quantum mechanical model — is used much more frequently.
A Mathematical Model
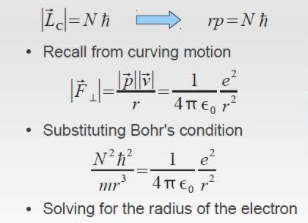 As discovered by Neil Bohr, the radius can be derived from using the angular momentum equation. The electron in only allowed to live in certain levels. Thus the radius is calculated.
As discovered by Neil Bohr, the radius can be derived from using the angular momentum equation. The electron in only allowed to live in certain levels. Thus the radius is calculated.
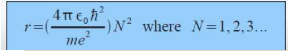
The total energy is also calculated through using this model.
A Computational Model
Here is a visualization of the Bohr Model, and its graph of Energy (eV), Kinetic Energy, and Potential Energy. This visualization shows how the electrons jump from level to level according to the Bohr Model.
Examples
Be sure to show all steps in your solution and include diagrams whenever possible
Simple
How much energy in electron volts is required to ionize a hydrogen atom (that is, remove the electron from the proton), if initially the atom is in the state N = 3? Here we can use the formula for the hydrogen atom which is E = 13.6/N^2 E = 13.6/N^2 E = 13.6/3^2 E = 1.51 Joules
Middling
Hydrogen has been detected transitioning from the 101st to the 100th energy levels.What is the radiation’s wavelength? Where in the electromagnetic spectrum is this emission? In order to solve this, all that is necessary is to substitute the formulas for Hydrogen atom that are derived from the Bohr Model.

Difficult
Connectedness
- How is this topic connected to something that you are interested in?
This topic is quite interesting as it is the initial introduction to quantum physics, which I find particularly interesting. Although, this model has had shortcomings, it is one of the most successful models of its time. It has many features that are used in the actual model for quantum physics.
- How is it connected to your major?
- Is there an interesting industrial application?
Bohr's discovery of the quantum leap is, in many ways, the most shining example of the consequences which Bohr's model of the atom has had for modern society. In effect, Bohr's notion that atoms emit light quanta with very specific energies is behind many of the technologies on which we, as humans, depend in our daily lives."Laser technology depends entirely on the principles behind Bohr's model of the atom because laser light is produced by quantum leaps. Quantum leaps between specific energy levels mean that the light has a specific frequency and wavelength and can therefore also be used to measure time and length very precisely. "Even though we cannot use Bohr's theory to make accurate calculations, we can use it as a conceptual framework.
http://scitech.au.dk/en/roemer/apr13/bohrs-model-of-the-atom-explains-science-in-everyday-life/
History
The first successful model of hydrogen was developed by Bohr in 1913, and incorporated the new ideas of quantum theory. Neil Bohr explained the emission spectra of hydrogen by improving on the Rutherford model of the atom. Initially, Rutherford's planetary model predicted a continuous spectrum of light from hydrogen. However, Bohr corrected for this by proposing that the translational angular momentum of the electron can be quantized. Although this model is not entirely correct, it has many features that are and is therefore used in physics.
Put this idea in historical context. Give the reader the Who, What, When, Where, and Why.
Shortcomings of the Bohr Model
The Bohr Model is an important predecessor to the current quantum mechanical models of the atom. However, there are some characteristics of the Bohr model, which are not entirely correct. The actual quantization rules in a hydrogen atom are much more complex than those that are assumed in the Bohr Model. The translational angular momentum in ground state which is N = 1, is 0 not h and for the next higher state of N = 2, the z component of translational angular momentum can either be 0 or h. Other issues with the Bohr Model include that it violates the Heisenberg Uncertainty Principle because it considers electrons to have both a known radius and orbit while they don't. It also makes poor predictions regarding spectra of larger atoms, and does not predict the relative intensities of spectral lines.
It does not explain the Zeeman Effect.
http://chemistry.about.com/od/atomicstructure/a/bohr-model.htm
See also
Are there related topics or categories in this wiki resource for the curious reader to explore? How does this topic fit into that context?
Further reading
Matter and Interactions I Modern Mechanics 4th Edition Chapter 11.10
External links
Simulation of Bohr Model: https://phet.colorado.edu/en/simulation/legacy/hydrogen-atom
References
This section contains the the references you used while writing this page