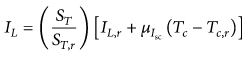The Photovoltaic Effect
Created by Andrew Kresses
The photovoltaic effect is the creation of electric current or voltage through a material upon exposure to light. It is a physical and chemical phenomenon in response to the transfer of energy from photons.

The Main Idea
The photovoltaic effect is directly related to The Photoelectric Effect, but they are different processes. When light strikes a material surface, the electrons present in the valence band absorb the energy from the photons and, being excited, jump to the conduction band and become free. The material is composed of crystallized atoms that are ionized and create an electric imbalance, that helps to drive the electrons. These highly excited electrons diffuse and some reach a junction where they are accelerated into a different material by an electrode. This generates an electric current, so some of the light energy can be converted into electric energy. This current can then be used to power an appliance.
Application
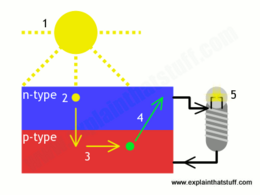
The photovoltaic effect is the evacuation of from a material due to the energy from photons in an incident light. We can harness the energy from this reaction with photovoltaic cells. This is because of the design on the photovoltaic or solar cell. The most basic of these cells are designed merely with two layers of a metalloid and an electrode. The top, or p-type layer of metalloid is generally composed of Boron doped silicon. This is because the added Boron creates a dearth of electrons on the top layer. The bottom, or n-type layer of the cell is usually made of a Phosphorous doped silicon. This creates a highly negative layer, which in conjunction with the positive p-type layer, creates a charge gradient that allows for the movement of free electrons and is the basis for photovoltaic cells.
Where the p-type and n-type layers meet, they form an electromagnetically stable barrier between the two layers that only allows electrons to flow from the p-type layer to the n-type layer. This allows electrons to flow into the n-type layer through the p-n junction boundary. The free electrons then return to the p-type layer by flowing through the electrode. This flow of electrons is what we call electricity, and it allows for the cyclical nature of the photovoltaic cell while also powering our technology.
Electrode Configuration
Forward Bias
With a forward bias, the p-type layer is connected to the positive terminal of the electrode, and the n-type layer is connected to the negative side. Reducing depletion width occurs because of the shrinking charge profile, as fewer dopants are exposed with increasing forward bias. When the photovoltaic cell is connected in this manner, the holes from the p-type region and the electrons in the n-type region are forced toward the junction. This reduces the width of the buffer zone. The positive charge of the p-type material repels the holes, while the negative energy of the n-type material repels the electrons.
Reverse Bias
In order to switch to a reverse bias, you simply swap the position of the electrode terminals. If a cell is reverse-biased, the voltage at the cathode is comparatively higher than the anode. Therefore, no current will flow until the depletion zone breaks down. Because the p-type layer is connected to the negative terminal, the "holes" in the p-type material are pulled away from the junction, and because the n-type layer is connected to the positive terminal, the electrons are pulled away from the junction. This results in the growth of the depletion zone. This increases the voltage barrier causes a high resistance to the flow of electricity, thus allowing very little current to cross what is now a functional insulator, the p–n junction.
Light Generated Current
This expression defines the light-generated current in a photovoltaic cell, IL, as a function of solar radiation and the temperature of the cell. ST represents the intitial solar radiation, and ST,r denotes the reflected solar radiation. Tc and Tc,r are the cell temperatures before and after irradiation. IL,r is light-generated current at reference conditions, and μIsc is coefficient of temperature at short circuit current. The initial cell temperature, Tc, can be computed from the ambient reference temperature.
History
The photovoltaic effect was discovered by a French physicist, Edmund Bequerel, in 1839, who found that certain materials would produce small amounts of electric current when irradiatedt. The first photovoltaic module was built by Bell Laboratories in 1954. It was billed as a solar battery and was mostly just a curiosity piece as it was too expensive to gain widespread use.
This trend still continues today as solar power technology is expensive and inefficient.
Connectedness
|-
This topic should be very important to all of us because we as a species are very quickly exhausting all of our non-renewable energy resources, and photovoltaic technology may be the solution. This topic is particularly interesting to me because I am a Materials Science and Engineering student. This scientific phenomena is heavily involved with the structure of the different materials involved. I am actually looking at researching next generation photovoltaic materials in the spring, so this project was a good study/introduction into the topic.
See Also
References
"P-N Junction." Hyper Physics. Georgia State, n.d. Web. 06 Dec. 2015.
Knier, Gil. "How Do Photovoltaics Work?" National Aeronautics and Space Administration. NASA Science, 2002. Web. 06 Dec. 2015.
Jakhrani, Abdul Quayoom. "An Improved Mathematical Model for Computing Power Output of Solar Photovoltaic Modules." International Journal of Photoenergy. Hindawi Publishing Corporation, 17 Mar. 2014. Web. 4 Dec. 2015.
Wikipedia contributors. "Photovoltaics." Wikipedia, The Free Encyclopedia. Wikipedia, The Free Encyclopedia, 3 Dec. 2015. Web. 6 Dec. 2015.