Thermal Energy
Thermal energy is energy possessed by an object or system due to the movement of particles within the object or the system.
The Main Idea
All objects are made up of numerous particles or molecules. The constant, random motion of these atoms or molecules is what we associate thermal energy with. Thermal energy is a component of internal energy, but is unrelated to the vibrational and rotational energy of a solid's atoms.. When we say change of "thermal" energy, we mean that it is that part of the internal energy that is associated with a temperature change. Thermal energy is quantified using temperature. This quantification describes the approximate average thermal kinetic energy present in all of the atoms or molecules in the system. In many situations it isn't possible to say how much of the internal energy is thermal, but if the heat capacity is known, we can use a thermometer to measure a change in the thermal energy.
A Mathematical Model
Change in thermal energy can be calculated by using the following mathematical equation. [math]\displaystyle{ {Q = m*C*dT} }[/math] where [math]\displaystyle{ {Q} }[/math] is the thermal energy in Joules, [math]\displaystyle{ {m} }[/math] is the mass of an object in grams, [math]\displaystyle{ {C} }[/math] is the object's specific heat capacity, and [math]\displaystyle{ {dT} }[/math] is the change in object's temperature in Celsius.
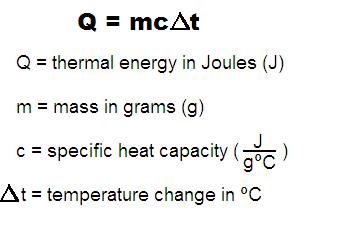
Temperature
In order to quantify thermal energy, a measurement of the average random energy of the atoms or molecules in a system is measured. This value is referred to as temperature. In order to measure temperature, a thermometer is utilized. The most well known representation of a thermometer is a thin glass column filled with either mercury or an alcohol. When a thermometer is placed into a system and time passes, the average kinetic energies of the system and the thermometer become the same. The change in thermal energy causes the alcohol or mercury to expand or compress. The temperature is then measured on a scale of Celsius or Kelvin. One Degree Kelvin is equivalent to approximately [math]\displaystyle{ 1*10e-23 }[/math]
The Kinetic Molecular Theory of Matter
The Idea of Thermal energy is derived from the Kinetic Molecular Theory of Matter. This theory describes why matter exists in different phases and provides a description of the interactions and properties of atoms through the use of ideas that are generally applied to larger systems. According to the Kinetic molecular Theory, all matter is comprised of lots of smaller molecules or atoms that are constantly moving. The type of motion of these particles is a result of the thermal energy present and determines whether the substance is in a gaseous, liquid, or solid state. When energy is introduced or lost from a material, the resulting change in motion of the individual particles can cause a phase change occur for the substance. There are small spaces between the atoms that make up matter and as the thermal energy of a substance increases, these spaces get progressively larger and begin to overcome the intermolecular forces present.
Examples
Simple
500g of water was heated from the initial temperature of 20°C to 50 °C. What is the change in thermal energy of water? (Heat capacity of water is 4.2J/g*°C)
1. List given things and equation
[math]\displaystyle{ Q = m*C*dT }[/math]
[math]\displaystyle{ Q=?, m=500g, C=4.2J/g*°C, dT={T}_{f}-{T}_{i}, {T}_{f}=50°C, {T}_{i}=20°C }[/math]
2. Plug the numbers into the equation and find the answer
[math]\displaystyle{ Q = 500g*4.2J/g*°C*(50°C-20°C) }[/math]
[math]\displaystyle{ Q = 63000J }[/math]
Moderate
400g of water with initial temperature of 90°C (specific heat of 4.2 J/g*°C) are poured into an aluminum pan whose mass is 800g with initial temperature of 20°C (specific heat of 0.9 J/g*°C). After a short time, what is the temperature of the water?
1. Write the equation and list the knowns and unknowns
[math]\displaystyle{ {{dE}_{water}+{dE}_{pan} = 0}, dE = m*C*dT }[/math]
[math]\displaystyle{ {m}_{water} = 400g,{m}_{pan} = 800g, {C}_{water} = 4.2J/g*°C {C}_{Aluminum} = 0.9J/g*°C, {T}_{i water} = 90°C, {T}_{i pan} = 20°C, {T}_{f} = ? }[/math]
2. Plug the numbers into the equation
[math]\displaystyle{ 0 = 400g*4.2J/g*°C*({T}_{f}-90°C) + 800g*0.9J/g*°C*({T}_{f}-20°C) }[/math]
3. Solve for [math]\displaystyle{ {T}_{f} }[/math] and find its value
[math]\displaystyle{ {T}_{f}=69°C }[/math]
Difficult
500g of water with initial temperature of 87°C (specific heat of 4.2 J/g*°C) are poured into an aluminum pan whose mass is 800g with initial temperature of 22°C (specific heat of 0.9 J/g*°C). Then you place the pan on a hot electric stove. While the stove is heating the pan, you stir the water doing 26000J of work, rising temperature of a system to 82.5 °C. How much energy transfer due to a temperature difference was there from the stove into the system consisting of the water plus the pan?
1. Write the equation and find the final temperature when water and pan first reached thermal equilibrium.
[math]\displaystyle{ {{dE}_{water}+{dE}_{pan} = W + Q}, dE = m*C*dT }[/math]
[math]\displaystyle{ W=0, Q=0, {m}_{water}=500g, {m}_{pan}=800g, {C}_{water}=4.2 J/g*°C, {C}_{aluminum}=0.9 J/g*°C, {T}_{1water}=87°C, {T}_{1pan}=22°C, {T}_{2}=? }[/math]
2. Plug the numbers into the equation and find the final temperature
[math]\displaystyle{ {dE}_{water}= 500*4.2*({T}_{2}-87) }[/math]
[math]\displaystyle{ {dE}_{pan}=800*0.9*({T}_{2}-22) }[/math]
[math]\displaystyle{ {dE}_{water}+{dE}_{pan}=0 }[/math]
3. Solve for [math]\displaystyle{ {T}_{f} }[/math] and find its value
[math]\displaystyle{ {T}_{f} = 70.404 }[/math]
4. Write the equation and list the knowns, repeating the steps above (final temperature above now becomes initial temperature)
[math]\displaystyle{ {{dE}_{water}+{dE}_{pan} = W + Q}, dE = m*C*dT }[/math]
[math]\displaystyle{ W=26000J, Q=?, {m}_{water}=500g, {m}_{pan}=800g, {C}_{water}=4.2 J/g*°C, {C}_{aluminum}=0.9 J/g*°C,{T}_{2}=70.404°C, {T}_{3}=82.5°C }[/math]
5. Plug the numbers into the equation
[math]\displaystyle{ 500*4.2*(82.5-70.404)+800*0.9*(82.5-70.404) = 26000 + Q }[/math]
6. Solve for Q and find its value
[math]\displaystyle{ Q = 8110J }[/math]
Real World Application
Thermal energy is an phenomenon that is present in all aspects of life. From the the sun to an ice cube, all contain thermal energy. In order to convert thermal energy to other, more useful forms an engine must be used. For example, steam carries a large amount of thermal energy and therefore is used to carry energy efficiently and carry out manufacturing processes. Similarly, one of the largest contributors to the determination of the phase in which substances exist, an aspect of physics that plays a large role in engineering. One of the interesting industrial application of thermal energy is industrial thermal energy storage. Thermal energy storage is a technology that stocks thermal energy by heating or cooling a storage medium so that the stored energy can be used at a later time for heating and cooling applications and power generation.
Thermal Equilibrium
Like all systems in nature, thermal energy works to reach equilibrium. When to substances of differing temperature come into contact, the substance of greater thermal energy will do microscopic work on the other substance until the two are at the same temperature. Atoms of the higher energy substance will collide will those of the lower energy substance at the boundary. Kinetic energy will be transferred and distribute throughout. Given enough time, this process of propagation of kinetic energy will continue until equilibrium is reached. The transfer of thermal energy is referred to as heat.
History
Thermal energy was discovered by a man named James Joule in the 1840s. Careful experiments show that the temperature increase of an object and its surroundings due to friction is directly related to the amount of mechanical energy lost. Joule carried one of the most famous experience demonstrating this fact. Joule hung some weights from pulleys so that as they fell, they turned a paddle-wheel apparatus immersed in a bucket of water. The friction between the paddles and the water raised the water’s temperature by an amount that was directly proportional to the distance that the weights fell. In our modern system of units, Joule found that raising the temperature of a kilogram of water by one degree Celsius required a loss in mechanical (gravitational) energy of approximately 4200 joules. Joule therefore proposed that this mechanical energy is not actually lost, but converted into a new type of energy: thermal energy, which manifests itself as an increase in temperature.
See also
Further reading
Matter and Interactions By Ruth W. Chabay, Bruce A. Sherwood - Chapter 7
http://physics.weber.edu/schroeder/eee/chapter3.pdf
http://p3server.pa.msu.edu/coursewiki/doku.php?id=183_notes:internal_energy
External links
http://www.eschooltoday.com/energy/kinds-of-energy/what-is-thermal-energy.html
http://study.com/academy/lesson/what-is-thermal-energy-definition-examples.html
https://www.chem.tamu.edu/class/majors/tutorialnotefiles/kinetic.htm
References
Matter and Interactions By Ruth W. Chabay, Bruce A. Sherwood - Chapter 7
