Electronic Energy Levels and Photons
Short Description of Topic
The Main Ideas
The Quantized Nature of Electronic Energy Levels
Electrons can be excited by absorbing energy from photons. Electrons can only be excited to certain electronic energy levels. Each electronic energy level is a number that represents the sum of the kinetic and potential energy (K+U). Because the electronic potential energy between the positive protons in the nucleus and the surrounding negative electrons will always be negative, the value of K+U will be negative. Because electrons are only stable at those energy levels, an electron can only absorb certain quantized energies from photons. Once the electron absorbs a photon, it is excited by the energy. After the electron is excited, it drops down and releases a photon with the energy difference between the two energy levels. It can drop to any energy level below it, and thus the resulting photons can be of several energies. If the photon gained is the the opposite of the K+U value for the energy level, then the electron is said to have been ionized. The ionization energy of an atom is the energy needed to ionize an electron that is at rest.
The Nature of a Photon
A photon falls neither in the category of a particle nor in the category of a wave. A photon behaves like a particle with a velocity, however it has no mass, and ceases to exist once its energy is absorbed. It can be created or destroyed at anytime, and thus cannot truly be considered as being a particle. It can be imagined as a elementary package of energy. It is a product of the wave-particle duality of light, which states that light behaves both as a particle was well as a wave. The relationship between the frequency of the wave of light and the energy contained in the photon can be described using Planck's Constant.
A Mathematical Model of the Bohr Hydrogen Atom
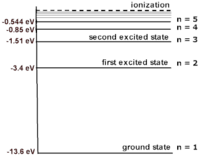
For example, the electronic energy levels for a hydrogen atom can be modeled by the equation: [math]\displaystyle{ {\frac{-13.6}{N^2}} = {K+U} }[/math] where N is the energy level. N=1 is the rest energy level; N=2 is the the first excited energy level; and N=3 is the second level, etc. This formula gives energy levels in terms of electron volts (eV). If you substitute values of N into the equation you can build the atom shown. As the value of N increases, the space between each energy level decreases. The energy difference between the rest energy level and the first excited energy level is the largest. Because the energy of the rest energy level is -13.6 eV, the ionization energy of an electron at rest in a hydrogen atom is 13.6 eV. In other words, if the electron at rest absorbs a photon with 13.6 eV, the electron is "freed" from the atom. In this atom, the difference between the the first (-13.6 eV) and second (-3.4 eV) energy level is 10.2 eV. This means that a photon needs to have a minimum energy of 10.2 eV to be absorbed by the electron and excite it.
A Computational Model
How do we visualize or predict using this topic. Consider embedding some vpython code here Teach hands-on with GlowScript
Examples
Simple
Middling
Difficult
Connectedness
- How is this topic connected to something that you are interested in?
- How is it connected to your major?
- Is there an interesting industrial application?
History
Put this idea in historical context. Give the reader the Who, What, When, Where, and Why.
See also
Further reading
Books, Articles or other print media on this topic
External links
http://physics.about.com/od/quantumphysics/f/quantumoptics.htm http://dev.physicslab.org/document.aspx?doctype=3&filename=atomicnuclear_bohrmodelderivation.xml