Potential Difference in an Insulator
Potential difference is defined as a scalar quantity that measures the difference of energy per unit charge. This page will not go over how to calculate electric potential in a conductor (because other pages cover this topic), but rather, how to find the electric potential in an insulator given the potential difference in a vacuum.
Potential Difference
Although this section will not go in depth into how to calculate potential difference, the following analysis requires the knowledge that potential difference equals the dot product of the electric field vector and distance vector between two points. Understanding that potential difference is dependent on the distance between two points is an important prerequisite to comprehending how to find the potential difference inside an insulator.
Net Electric Field Inside an Insulator
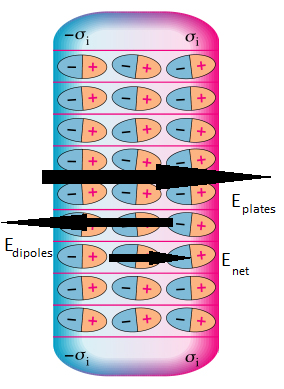
Between two points inside a metal object in equilibrium, the potential difference is zero. However, this is untrue for an insulator that is polarized by an applied electric field (such as that inside a capacitor). In order to quantitatively predict the potential difference between two points in an insulator, we first must understand how this applied electric field polarizes molecules in the surrounding objects. Typically, this electric field creates induced dipoles inside the insulating materials. These induced dipoles contribute their own electric field to the net field. Because dipoles create such a unique pattern of electric field, it is slightly more complex to find the electric field and potential difference inside the insulator.
The net electric field inside the insulating material is the sum of the applied electric field (often due to a capacitor) and the electric field produced by the induced dipoles. The field from the induced dipoles always points in the direction opposite to the applied electric field (as shown in Diagram 1). Consequently, the net electric field is in the direction of the applied field/capacitor, but it is weaker in magnitude.
Dielectric Constant
When solving for the potential difference in an insulator, we define the constant K as the dielectric constant. This quantity represents the amount by which the net electric field is "weakened" due to the induced dipoles. It is a value typically known through previous scientific experimentation (specifically, measuring the effect of an insulator on the potential difference between two charged objects).
A Mathematical Model for the Electric Field Inside an Insulator
[math]\displaystyle{ \vec {E}_{insulator} = \frac{\vec{E}_{applied}}{K} }[/math]
The dielectric constant K is always bigger than one if an insulator is present because the induced dipoles in the polarized insulator always weaken the net electric field. When an insulating substance is easy t polarize, K will be large because the induced dipoles will create a weaker net electric field. If there is no insulating material between the charged objects (the space is a vacuum), K equals one.
A Computational Model
How do we visualize or predict using this topic. Consider embedding some vpython code here Teach hands-on with GlowScript
Relating Electric Field to Potential Difference
Because the relationship between the electric field and potential difference is proportional, potential difference will also decrease by a value K. That is, placing an insulator in between two charged objects like the plates of a capacitor also decreases potential difference across the insulator. It is important to note that if the insulator does not fill the gap between objects, the electric field and potential difference inside the insulator are still reduced by a factor K. However, the areas that are not filled by the insulator are not affected since the electric field inside the insulator is negligibly small.
A Mathematical Model
E2 - E1 = Q - W
Second Law
The second law states that there is another useful variable of heat, entropy (S). Entropy can be described as the disorder or chaos of a system, but in physics, we will just refer to it as another variable like enthalpy or temperature. For any given physical process, the combined entropy of a system and the environment remains a constant if the process can be reversed. The second law also states that if the physical process is irreversible, the combined entropy of the system and the environment must increase. Therefore, the final entropy must be greater than the initial entropy.
Mathematical Models
delta S = delta Q/T Sf = Si (reversible process) Sf > Si (irreversible process)
Examples
Reversible process: Ideally forcing a flow through a constricted pipe, where there are no boundary layers. As the flow moves through the constriction, the pressure, volume and temperature change, but they return to their normal values once they hit the downstream. This return to the variables' original values allows there to be no change in entropy. It is often known as an isentropic process.
Irreversible process: When a hot object and cold object are put in contact with each other, eventually the heat from the hot object will transfer to the cold object and the two will reach the same temperature and stay constant at that temperature, reaching equilibrium. However, once those objects are separated, they will remain at that equilibrium temperature until something else acts upon it. The objects do not go back to their original temperatures so there is a change in entropy.
Connectedness
- How is this topic connected to something that you are interested in?
- How is it connected to your major?
- Is there an interesting industrial application?
History
Thermodynamics was brought up as a science in the 18th and 19th centuries. However, it was first brought up by Galilei, who introduced the concept of temperature and invented the first thermometer. G. Black first introduced the word 'thermodynamics'. Later, G. Wilke introduced another unit of measurement known as the calorie that measures heat. The idea of thermodynamics was brought up by Nicolas Leonard Sadi Carnot. He is often known as "the father of thermodynamics". It all began with the development of the steam engine during the Industrial Revolution. He devised an ideal cycle of operation. During his observations and experimentations, he had the incorrect notion that heat is conserved, however he was able to lay down theorems that led to the development of thermodynamics. In the 20th century, the science of thermodynamics became a conventional term and a basic division of physics. Thermodynamics dealt with the study of general properties of physical systems under equilibrium and the conditions necessary to obtain equilibrium.
See also
Are there related topics or categories in this wiki resource for the curious reader to explore? How does this topic fit into that context?
Further reading
Books, Articles or other print media on this topic
External links
Internet resources on this topic
References
https://www.grc.nasa.gov/www/k-12/airplane/thermo0.html http://hyperphysics.phy-astr.gsu.edu/hbase/thermo/thereq.html https://www.grc.nasa.gov/www/k-12/airplane/thermo2.html http://www.phys.nthu.edu.tw/~thschang/notes/GP21.pdf http://www.eoearth.org/view/article/153532/
-cbrogan7