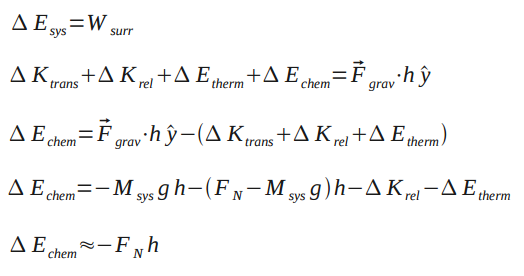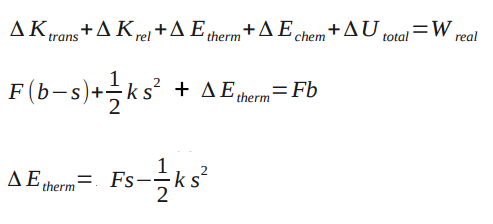Real Systems
This page describes real systems and how they can be used to model certain aspects of a system's motion.
The Main Idea
A real system is a system in which every part of the system is modeled separately, allowing for the internal behaviors of the system to be analyzed in addition to the system's motion through its environment.
In Point Particle Systems, forces are analyzed as though they act directly on the system's center of mass, and only the translational kinetic energy of a system can change. Through week 9, we have been most bodies as point particles, as we have been primarily interested in their translational motion. However, we may now encounter problems where we want to analyze the internal behaviors of systems as well. For example, we may want to calculate a change in a system's Translational, Rotational and Vibrational Energy, Potential Energy, or Thermal Energy. In a real system, the point of application of each force must be considered. Furthermore, when calculating the work done by a force, the distance over which the force is applied is not the distance traveled by the system's center of mass, but rather the distance traveled by the force's point of contact. These two key differences lead to a mathematical model that can be more complicated than that of a point particle system, but yields insights about the internal behavior of the system.
A Mathematical Model
The mathematical concepts used to analyze point particle systems depend on the system. Often, the work-energy theorem (see Work/Energy) is used. This section explains how to use the work-energy theorem for a point particle system because this is the concept that varies the most significantly from its application to point particle systems.
The work done on a real system should be calculated separately for each force acting on it because each force might be exerted over a different distance due to the geometry of the system. The work done on a real system by each force is defined as
[math]\displaystyle{ W = \int \vec{f} \cdot d \vec{r} }[/math],
where [math]\displaystyle{ \vec{r} }[/math] is the position of the point on the system on which the force acts.
Let us assume that the force acting on the system is constant, so that we can get rid of the integral, and that the force acts in the direction of the system's motion, so that we can replace the dot product with regular multiplication. This will be the case for most real system problems involving work. With these assumptions, the work done on a part of a real system by a force is given by
[math]\displaystyle{ W = f * d }[/math]
where [math]\displaystyle{ F_{net} }[/math] is the magnitude of the net force acting on the particle and [math]\displaystyle{ d }[/math] is the distance over which the force is exerted. For real systems, that is the distance traveled by the point of contact of the force, which may not be the same as the distance traveled by the system's center of mass, depending on the movement of the systems' different parts. This contrasts with point particle systems, where the distance over which the force is exerted is simply the displacement of the system's center of mass.
The work-energy theorem states that work done on a system increases that system's energy. For real systems, this is the system's total energy. Since each force's work must be found separately in a real system, remember to sum the values of the work done by the different forces acting on the system.
In other words, for a real system,
[math]\displaystyle{ \Delta E = \sum_i f_i * d_i }[/math].
The equation above is the basis for answering work/energy questions using real systems. It is important to remember that when modeling a system as a real system, calculating the work done on the system as described above finds all of the work done on the system- both translational kinetic energy and internal types of energy.
A Computational Model
Most computer simulations treat systems as real systems, modeling each part of a system separately. This is because Iterative Prediction makes keeping track of each part of the system separately easy even when it might be difficult to do so analytically. Consider this simulation of the two-mass system described in example problem 4 of the point particle system page. If you click "view source" on the top left corner, you will see that the process of iterative prediction is performed separately for each of the two masses- they each have their own position vectors, and the forces acting on each are calculated. there is no need to treat them as a single particle and thereby lose information about their internal behavior- in this case, their oscillations.
Examples
In order to better display the difference of Real Systems from Point Particle Systems, the examples done here will be the same examples done from Point Particle Systems.
Jumper Model (Simple)
Problem: You jump up so that your center of mass has moved a distance h. How much chemical energy did you expend?
From the Point Particle System analysis, we know that and
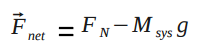
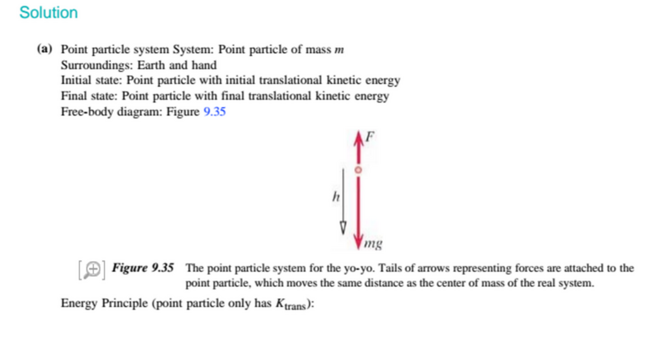
System: Person Surroundings: Earth+Floor
Initial State: Crouched down
Final State: Extended and moving with speed v
Assuming negligent change in thermal energy and relative kinetic energy, the change in thermal energy is approximately equal to the normal force multiplied by height.
Yo-Yo (Middling)
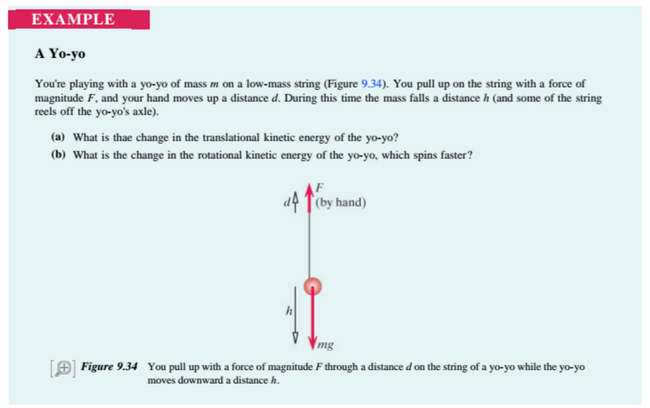 link title(Chabay)
link title(Chabay)
Step 1: Solve for translational kinetic energy using the Point Particle System
(The equation for translational kinetic energy here is different than that in Point Particle Systems, so the derivation has been provided.)
Step Two: Solve for rotational kinetic energy using a Real System
Here is where the true difference between Real and Point Particle Systems can be seen. In the Point Particle system, there is no value to account for the change of rotational kinetic energy from the work done the hand. By changing the Work equation to 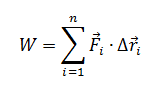
, the rotational kinetic energy can now be found.
Spring In a Box (Difficult)
Suppose a thin box contains a ball of clay with the mass M connected to a relaxed spring with a stiffness ks. The masses of the box and the spring are negligible. It is initally at rest, and then a constant force of F. The box moves a distance b and the spring stretches a distance s so that the clay sticks to the box. What is the change in thermal energy of the clay after colliding with the wall of the box?
From the analysis of the Point Particle Systems of the Spring in a Box, we know that . Because the system is a spring, we also know that
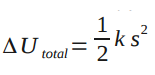
Assuming there is no relative kinetic energy (none based on diagram) and no change in chemical energy (there is no change in substance), the change in thermal energy of the clay can be found. Finding the change in thermal energy is important because you can determine whether there was enough energy to change the temperature of the clay or whether there is enough energy given off by the clay to change the temperature of a surrounding substance by a degree. Problems like this show the importance of analyzing real systems versus point particle systems.
Connectedness
How is this topic connected to something that you are interested in?
This topic interests me because from one single system you can mathematically determine the other forms of energy that can occur in various physical interaction. From the other forms of energy, you can determine whether there is enough energy to maybe change the temperature of another substance via thermal energy or even change the substance that is in the system given a big enough change in chemical energy.
How is it connected to your major?
As a chemical engineering major, the application of Real Systems is largely used for the majority of mathematics in my major dealing with energy balances. From only analyzing a system from a point particle method, one would only be able to find the change in the translational kinetic energy. In my major, it is very important to consider the entire system in order to find important values such as the change in thermal and kinetic energy because these values are often associated with the amount of work and heat produced in many chemical engineering processes.
Is there an interesting industrial application?
There is an absolute overload of interesting industrial applications for the analysis of real systems. In fact, the analysis of real systems in terms of energy balances is the entirety of what I've done in my chemical engineering classes thus far (I am currently a second year). There are many interesting (depending on your taste) uses of the real system analysis on a multitude of different turbines and chemical reactors.
See also
Further reading
http://p3server.pa.msu.edu/coursewiki/doku.php?id=183_notes:pp_vs_real
External links
References
Chabay, Ruth W., and Bruce A. Sherwood. "9." Matter & Interactions. N.p.: n.p., n.d. N. pag. Print.
Wiki Commons Picture
--Nfortingo3(talk) 19:26, 28 November 2015 (EST)