Crystalline Structures
Crystalline structures are the foundation of solid-state materials, describing the orderly arrangement of atoms, ions, or molecules in three-dimensional space. These structures are characterized by their repeating patterns, known as lattices, which extend throughout the material. The study of crystalline structures is essential in understanding the physical and chemical properties of solids, including their strength, conductivity, thermal behavior, and optical properties.
Bravais lattices
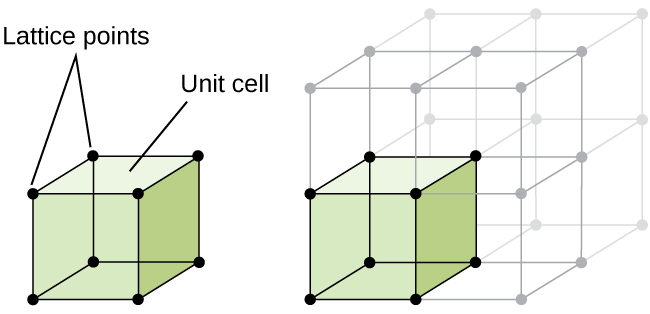
At the heart of crystallography is the concept of the unit cell—the smallest repeating unit that defines the symmetry and geometry of the entire lattice. Unit cells can take on a variety of configurations, each classified under the Bravais lattices, which represent the 14 unique ways atoms can be arranged in space. We will only cover 3 of the main configurations.
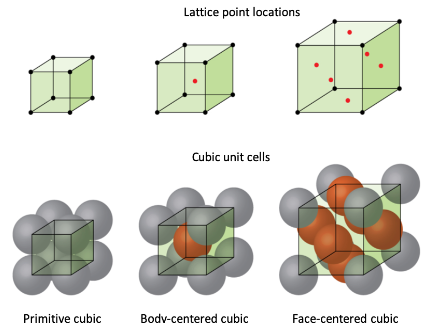
The main types of configurations that a Bravais lattice can take are: Primitive, Body-Centered Cubic (BCC), and Face-Centered Cubic (FCC). These configurations describe how atoms are arranged within a unit cell and are essential for understanding the structure and properties of crystalline materials. Among these, Primitive is the least efficient and weakest in terms of packing and structural strength, as its atoms are arranged only at the corners of the unit cell, leaving significant empty space. BCC, with an additional atom at the center of the unit cell, is stronger and has a higher packing efficiency than Primitive. However, FCC is the most efficient and stable configuration, with atoms occupying the corners and centers of all cube faces, resulting in the highest packing density and contributing to greater ductility and strength in materials that adopt this structure. These differences in packing and arrangement significantly influence the material properties, including density, strength, and thermal and electrical conductivity.
Primitive (Simple Cubic):
In this configuration, atoms are located only at the corners of the unit cell. Each corner atom is shared with adjacent unit cells, resulting in one net atom per unit cell. The packing efficiency is low, making this arrangement relatively rare in nature.
Example: Polonium, Caesium Chloride
Body-Centered Cubic
In addition to the corner atoms, there is a single atom located at the center of the unit cell. This arrangement increases the packing efficiency compared to the primitive structure but still leaves some empty space within the lattice.
Example: Iron, Tungsten, Potassium
Face-Centered Cubic
Atoms are present at each corner and the centers of all the cube faces. This configuration has the highest packing efficiency among the cubic structures (74%), leading to dense and stable crystal arrangements.
Example: Aluminum, Copper, Gold, Salt
Impurities
Impurities are atoms or molecules that are not part of the ideal crystal lattice but are present in the material. These can be either intentionally introduced or occur naturally during the crystal's formation. Impurities play a crucial role in determining the material's properties and are often exploited in applications like semiconductors and metallurgy.
Types of Impurities
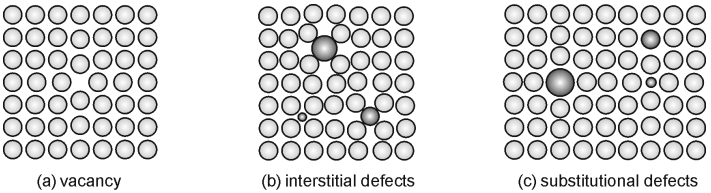
Substitutional Impurities:
Foreign atoms replace the host atoms in the crystal lattice.
Example: In brass, zinc atoms substitute copper atoms.
Interstitial Impurities:
Smaller foreign atoms occupy the spaces (interstitial sites) between the host atoms in the lattice.
Example: Carbon atoms in the interstitial sites of iron, forming steel.
Point Defects/Vacancy:
Includes vacancies, where an atom is missing from the lattice, or foreign atoms that disrupt the crystal's regularity.
Effects of Impurities
Mechanical Properties: Impurities can strengthen a material by hindering dislocation movement (e.g., in alloys like steel).
Electrical Properties: In semiconductors, impurities (dopants) drastically alter conductivity. For example, adding boron to silicon creates a p-type semiconductor.
Optical Properties: Impurities can absorb or scatter light, influencing transparency or color.
Applications
Doping in Semiconductors: Controlled addition of impurities like phosphorus or boron to silicon enables the creation of Diodes or Transistors.
Alloying: Mixing metals with specific impurities enhances their strength, corrosion resistance, or workability such as Steel.
Dislocations
Sources
https://wisc.pb.unizin.org/minimisgenchem/chapter/types-of-unit-cells-body-centered-cubic-and-face-centered-cubic-m11q5/ https://creativecommons.org/licenses/by-nc-sa/4.0/
https://wisc.pb.unizin.org/chem103and104/chapter/types-of-unit-cells-primitive-cubic-cell-m11q4/ https://creativecommons.org/licenses/by-nc-sa/4.0/
https://www.uobabylon.edu.iq/eprints/publication_10_6164_84.pdf