Crystalline Structures
Bravais lattices
Bravais Lattices are the basic patterns that describe how points can be arranged in space to form a crystal lattice. Named after Auguste Bravais, who studied them in 1848, there are 14 unique Bravais lattices, each representing a distinct way to repeat a pattern in three dimensions. These lattices are grouped into seven crystal systems: cubic, tetragonal, orthorhombic, hexagonal, trigonal, monoclinic, and triclinic. Each lattice is defined by its symmetry and how it repeats in space, forming the backbone of all crystalline materials. Understanding Bravais lattices is important because they help explain how atoms are arranged in crystals and how this arrangement affects a material's properties like strength, conductivity, and optical behavior.
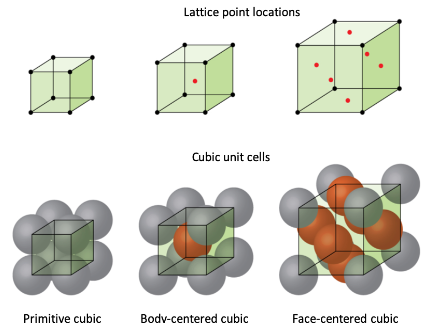
The main types of configurations that a Bravais lattice can take are: Primitive, Body-Centered Cubic (BCC), and Face-Centered Cubic (FCC). These configurations describe how atoms are arranged within a unit cell and are essential for understanding the structure and properties of crystalline materials. Among these, Primitive is the least efficient and weakest in terms of packing and structural strength, as its atoms are arranged only at the corners of the unit cell, leaving significant empty space. BCC, with an additional atom at the center of the unit cell, is stronger and has a higher packing efficiency than Primitive. However, FCC is the most efficient and stable configuration, with atoms occupying the corners and centers of all cube faces, resulting in the highest packing density and contributing to greater ductility and strength in materials that adopt this structure. These differences in packing and arrangement significantly influence the material properties, including density, strength, and thermal and electrical conductivity.
Primitive (Simple Cubic):
In this configuration, atoms are located only at the corners of the unit cell. Each corner atom is shared with adjacent unit cells, resulting in one net atom per unit cell. The packing efficiency is low, making this arrangement relatively rare in nature.
Example: Polonium, Caesium Chloride
Body-Centered Cubic
In addition to the corner atoms, there is a single atom located at the center of the unit cell. This arrangement increases the packing efficiency compared to the primitive structure but still leaves some empty space within the lattice.
Example: Iron, Tungsten, Potassium (K)
Face-Centered Cubic
Atoms are present at each corner and the centers of all the cube faces. This configuration has the highest packing efficiency among the cubic structures (74%), leading to dense and stable crystal arrangements.
Example: Aluminum, Copper, Gold, Salt